Error processing SSI file
Weekly Report: Influenza Summary Update
2007-2008 Influenza Season Week 18, ending May 3, 2008
(All data are preliminary and may change as more reports are received.)Synopsis
During week 18 (April 27 ?May 3, 2008), influenza activity continued to decrease in the United States.
- Seventy-seven (4.5%) specimens tested by U.S. World Health Organization (WHO) and National Respiratory and Enteric Virus Surveillance System (NREVSS) collaborating laboratories were positive for influenza.
- The proportion of deaths attributed to pneumonia and influenza has been above the epidemic threshold for 17 consecutive weeks.
- The proportion of outpatient visits for influenza-like illness (ILI) and acute respiratory illness (ARI) was below national and region-specific baseline levels.
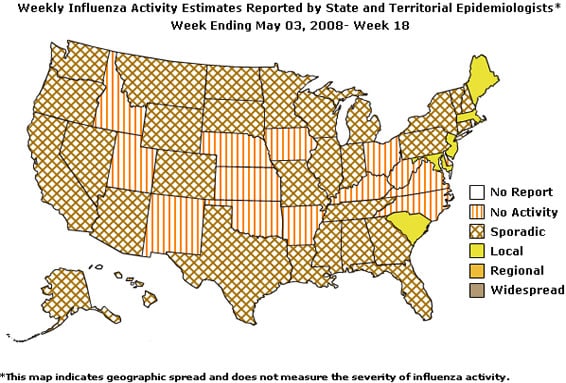
- Five states reported local influenza activity; 30 states, the District of Columbia and Puerto Rico reported sporadic influenza activity; and 15 states reported no influenza activity.
Region |
Data for current week | Data cumulative for the season | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sentinel Provider ILI* | DoD and VA ARI* | % pos. for flu† | # jurisdictions reporting regional or widespread activity‡ | A (H1) | A (H3) | A Unsub-typed | B | Pediatric Deaths | |
| Nation | Normal | Normal | 4.5 % | 0 of 51 | 2173 | 6086 | 19605 | 11093 | 69 |
| New England | Normal | Normal | 6.2 % | 0 of 6 | 97 | 260 | 1110 | 1207 | 8 |
| Mid-Atlantic | Normal | Normal | 6.8 % | 0 of 3 | 209 | 353 | 1955 | 1957 | 13 |
| East North Central | Normal | Normal | 9.9 % | 0 of 5 | 185 | 1425 | 630 | 590 | 8 |
| West North Central | Normal | Normal | 7.2 % | 0 of 7 | 107 | 248 | 3043 | 1776 | 6 |
| South Atlantic | Normal | Normal | 7.9 % | 0 of 9 | 352 | 1825 | 4786 | 1754 | 7 |
| East South Central | Normal | Normal | 6.9 % | 0 of 4 | 37 | 758 | 152 | 147 | 6 |
| West South Central | Normal | Normal | 3.2 % | 0 of 4 | 109 | 499 | 6001 | 1728 | 8 |
| Mountain | Normal | Normal | 4.7 % | 0 of 8 | 531 | 475 | 1003 | 1081 | 6 |
| Pacific | Normal | Normal | 6.8 % | 0 of 5 | 546 | 243 | 925 | 853 | 7 |
* Elevated means the % of visits for ILI or ARI is at or above the national or
region-specific baseline
?National data is for current week; regional data is for the most recent 3 weeks.
?Includes all 50 states and the District of Columbia
Laboratory Surveillance
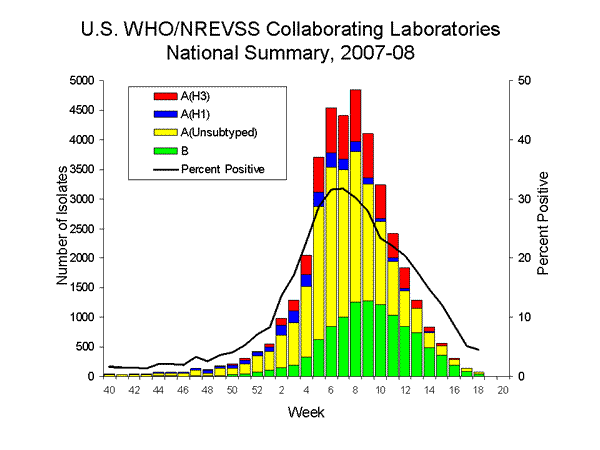
During week 18, WHO and NREVSS laboratories reported 1,714 specimens tested for influenza viruses, 77 (4.5%) of which were positive, including one influenza A (H1) virus, one influenza A (H3) virus, 30 influenza A viruses that were not subtyped, and 45 influenza B viruses.
Since September 30, 2007, WHO and NREVSS laboratories have tested a total of 213,835 specimens for influenza viruses and 38,957 (18.2%) were positive. Among the 38,957 influenza viruses, 27,864 (71.5%) were influenza A viruses and 11,093 (28.5%) were influenza B viruses. Eight thousand two hundred fifty-nine (29.6%) of the 27,864 influenza A viruses have been subtyped: 2,173 (26.3%) were influenza A (H1) viruses and 6,086 (73.7%) were influenza A (H3) viruses.
During the 2007-08 season, influenza A (H1), A (H3), and B viruses have co-circulated in the United States. Influenza A (H3) viruses have predominated during the season overall; however, the most commonly reported influenza virus has varied by week. From week 40 through week 3 (September 30, 2007 ?January 19, 2008) influenza A (H1) viruses were more frequently reported; from week 4 through week 12 (January 20 ?March 22, 2008), influenza A (H3) viruses were more commonly reported; and from weeks 13 through 18 (March 23 ?May 3, 2008), more influenza B than influenza A viruses were reported. The predominant virus has also varied by region. Influenza A (H3) viruses have been reported more frequently than A (H1) viruses in seven of the nine surveillance regions (East North Central, East South Central, Mid-Atlantic, New England, South Atlantic, West North Central, and West South Central), while influenza A (H1) viruses have predominated this season in two regions (Mountain and Pacific).
View WHO-NREVSS Regional Bar Charts | View Chart Data | View Full Screen
Composition of the 2008-09 Influenza Vaccine:
WHO and FDA have recommended that the 2008-09 trivalent influenza vaccine for the Northern Hemisphere contain A/Brisbane/59/2007-like (H1N1), A/Brisbane/10/2007-like (H3N2), and B/Florida/4/2006-like viruses. All three components have been changed from the 2007-08 Northern Hemisphere vaccine formulation. A/Brisbane/10/2007-like (H3N2) and B/Florida/4/2006-like viruses are currently included in the 2008 Southern Hemisphere vaccines. This recommendation was based on surveillance data related to epidemiology and antigenic characteristics, serological responses to 2007-08 vaccines, and the availability of candidate strains and reagents.
Antigenic Characterization:
CDC has antigenically characterized 794 influenza viruses [381 influenza A (H1N1), 193 influenza A (H3N2), and 220 influenza B viruses] collected by U.S. laboratories since September 30, 2007.
Influenza A (H1N1) [381]
- Two hundred sixty-one (69%) of the 381 viruses were characterized as A/Solomon Islands/3/2006-like, the influenza A (H1N1) component of the 2007-08 influenza vaccine for the Northern Hemisphere and the 2008 influenza A (H1N1) component for the Southern Hemisphere.
- Twenty (5%) of the 381 viruses showed somewhat reduced titers with antisera produced against A/Solomon Islands/3/2006.
- One hundred (26%) of the 381 viruses were characterized as A/Brisbane/59/2007-like. A/Brisbane/59/2007 is a recent genetic/antigenic variant which evolved from A/Solomon Islands/03/2006. An A/Brisbane/59/2007-like virus is the WHO recommended strain for the 2008-09 Northern Hemisphere vaccine formulation.
Influenza A (H3N2) [193]
- Forty-seven (24%) of the 193 viruses were characterized as A/Wisconsin/67/2005-like, the influenza A (H3N2) component of the 2007-08 influenza vaccine for the Northern Hemisphere.
- One hundred twenty-seven (66%) of the 193 viruses were characterized as A/Brisbane/10/2007-like. A/Brisbane/10/2007-like viruses are a recent antigenic variant which evolved from, but are antigenically distinct from, A/Wisconsin/67/2005-like viruses. A/Brisbane/10/2007-like virus is the recommended influenza A (H3N2) component for the 2008 Southern Hemisphere and 2008-09 Northern Hemisphere vaccines.
- Nineteen (10%) of the 193 viruses showed somewhat reduced titers with antisera produced against A/Wisconsin/67/2005 and A/Brisbane/10/2007.
Influenza B (B/Victoria/02/87 and B/Yamagata/16/88 lineages) [220]Victoria lineage [8]
- Eight (4%) of the 220 influenza B viruses characterized belong to the B/Victoria lineage of viruses.
o Six (75%) of these 8 viruses were characterized as B/Ohio/01/2005-like. The recommended influenza B component for the 2007-08 influenza vaccine is a B/Malaysia/2506/2004-like virus, belonging to the B/Victoria lineage. B/Ohio/01/2005 is a recent B/Malaysia/2506/2004-like reference strain.
o Two (25%) of these 8 viruses showed somewhat reduced titers with antisera produced against B/Ohio/01/2005 and B/Malaysia/2506/2004.
Yamagata lineage [212]
- Two hundred twelve (96%) of the 220 influenza B viruses characterized belong to the B/Yamagata lineage of viruses.
o Two hundred three (96%) of these 212 viruses were identified as B/Florida/04/2006-like, the recommended influenza B component for the 2008-09 Northern Hemisphere vaccine formulation.
o Nine (4%) of these 212 viruses showed a somewhat reduced titer with antiserum produced against B/Florida/04/2006.
These data indicate similarities and differences between a sample of circulating strains and this year's vaccine strains as determined by laboratory studies. Interim results from a study carried out with the Marshfield Clinic in Wisconsin found vaccine effectiveness of 58% against circulating influenza A (H3N2) viruses, based on data collected from Jan 21 through Feb 8, 2008. No vaccine effectiveness against influenza B viruses was found. No influenza A (H1N1) viruses were identified through Feb 8 and, thus, no vaccine effectiveness estimate is available for H1N1 viruses. These interim results suggest that vaccination provided substantial protection against H3N2 influenza-associated medically attended illness in the study population. Enrollment in this study concluded on March 28, 2008, and final results will be available later this year. Additional information on this study can be found at: http://www.cy118119.com/mmwr/preview/mmwrhtml/mm5715a1.htm
Antiviral Resistance:
In the United States, two groups of antiviral drugs have been approved by FDA for use in treating or preventing influenza virus infections. These two groups of antiviral drugs are: neuraminidase inhibitors (oseltamivir and zanamivir) and adamantanes (amantadine and rimantadine). A description of these drugs can be found at: http://www.cy118119.com/flu/protect/antiviral/index.htm.
Neuraminidase Inhibitor Antiviral Drugs: : So far this season, 1,474 influenza A and B viruses from the United States have been tested for antiviral resistance. One hundred one (8.3%) of 1,215 influenza A viruses tested, and 0 (0.0%) of 259 influenza B viruses tested have been found to be resistant to oseltamivir. Currently all of the resistant viruses are influenza A (H1N1) viruses, with 101 (11.1%) of 913 influenza A (H1N1) viruses tested exhibiting a genetic mutation that confers oseltamivir resistance. All tested viruses retain their sensitivity to zanamivir. Additional information on antiviral resistance can be found at: http://www.cy118119.com/flu/about/qa/antiviralresistance.htm
Adamantane Antiviral Drugs: Resistance to the adamantanes continues to be high among influenza A (H3N2) viruses with 364 (99.7%) of 365 influenza A (H3N2) viruses tested resistant to the adamantanes. Adamantane resistance among influenza A (H1N1) viruses has also been detected but at a lower level. Of 819 influenza A (H1N1) viruses tested, 88 (10.7%) were resistant to the adamantanes. The adamantanes are not effective against influenza B viruses. Since late January, influenza A (H3N2) viruses have predominated in the United States, and during week 18, 50% of influenza A viruses subtyped were A (H3N2).
Based on the level of oseltamivir resistance observed in only one influenza subtype, H1N1, persisting high levels of resistance to the adamantanes in H3N2 viruses, and the predominance of H3N2 viruses circulating in the United States during the 2007-08 season with co-circulation of influenza B viruses, CDC continues to recommend the use of oseltamivir and zanamivir for the treatment or prevention of influenza. Use of amantadine or rimantadine is not recommended. Guidance on influenza antiviral use can be found at: http://www.cy118119.com/mmwr/preview/mmwrhtml/rr5606a1.htm
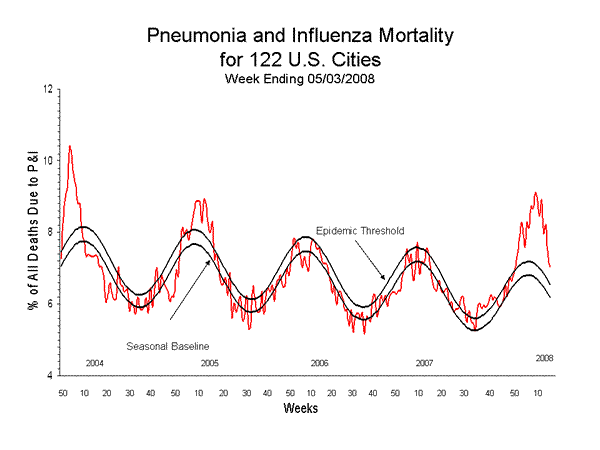
Pneumonia and Influenza (P&I) Mortality Surveillance
During week 18, 7.0% of all deaths reported through the 122 Cities Mortality Reporting System were reported as due to P&I. This percentage is above the epidemic threshold of 6.6% for week 18. Including week 18, P&I mortality has been above epidemic threshold for 17 consecutive weeks.
View Full Screen
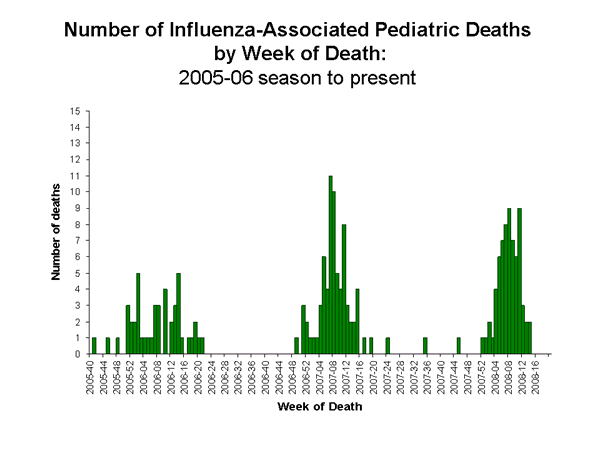
Influenza-Associated Pediatric Mortality
One influenza-associated pediatric death was reported to CDC during week 18 (NY). This death occurred during week 12 (March 16-22, 2008). Since September 30, 2007, CDC has received a total of 69 reports of influenza-associated pediatric deaths that occurred during the current season.
View Full Screen
Influenza-Associated Pediatric Hospitalizations
Laboratory-confirmed influenza-associated pediatric hospitalizations are monitored in two population-based surveillance networks: the New Vaccine Surveillance Network (NVSN) and the Emerging Infections Program (EIP). These two systems provide updates of surveillance data every two weeks. As a result of differing dates for initiating surveillance in the 2007-08 season, these updates occur on alternating weeks.
During November 4, 2007 to April 19, 2008, the preliminary laboratory-confirmed influenza-associated hospitalization rate for children 0-4 years old in the NVSN was 6.89 per 10,000.
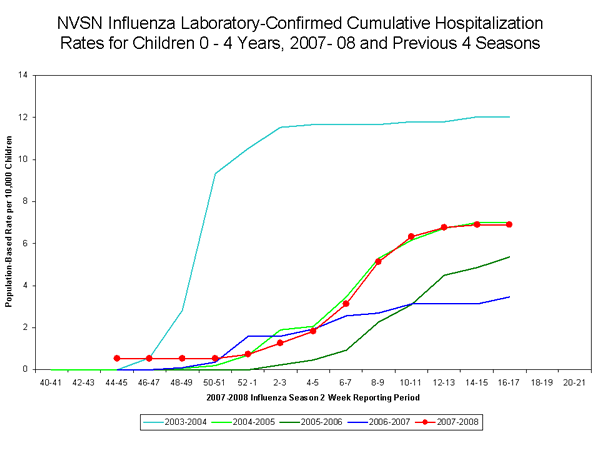
View Full Screen
During September 30, 2007 ?April 26, 2008, the preliminary laboratory-confirmed influenza-associated hospitalization rate reported by the EIP for children 0?7 years old was 1.50 per 10,000. For children aged 0-4 years and 5-17 years, the rate was 3.94 per 10,000 and 0.52 per 10,000, respectively.
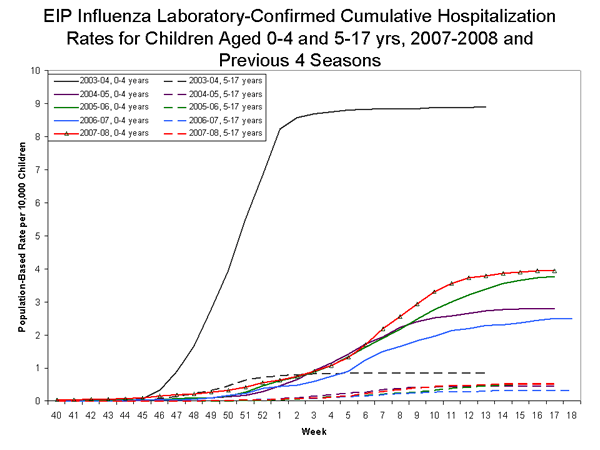
View Full Screen
Outpatient Illness Surveillance
Nationwide during week 18, 0.9% of outpatient visits reported through the U.S. Influenza Sentinel Provider Surveillance Network were due to influenza-like illness (ILI), which was below the national baseline of 2.2%. On a regional level, the percentage of outpatient visits for ILI ranged from 0.5% to 1.3%. All nine regions reported ILI below their region-specific baselines.
During week 18, 1.9% of patient visits to Department of Veteran抯 Affairs (VA) and Department of Defense (DoD) outpatient treatment facilities were for acute respiratory illness (ARI), which was below the national baseline of 3.2%. On a regional level, the percentage of visits for ARI ranged from 0.9% to 2.3%, and was below region-specific baselines in all nine regions. All five age groups reported ARI below their age-specific baselines.
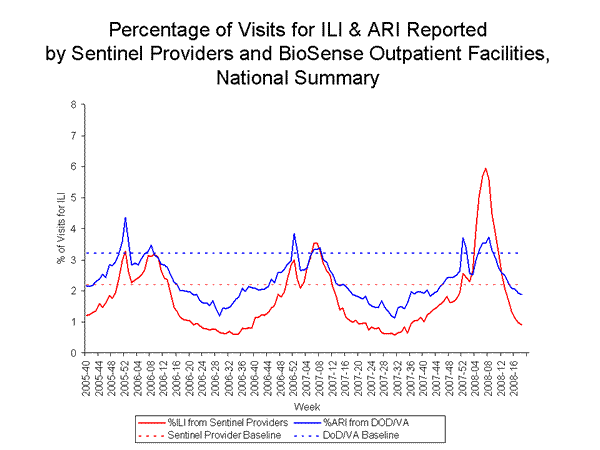
View Sentinel Providers Regional Charts | View Chart Data |View Full Screen
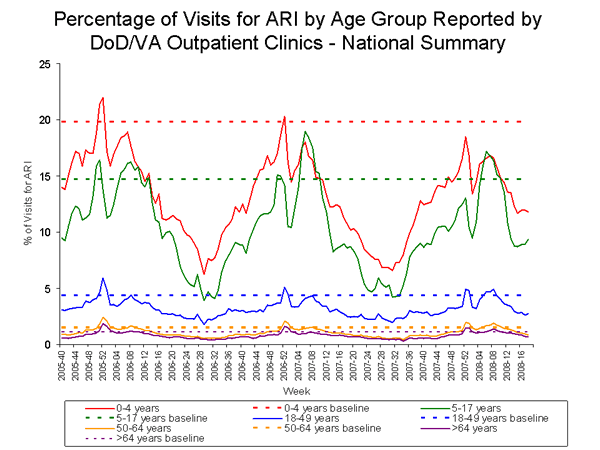
View Full Screen
Geographic Spread of Influenza as Assessed by State and Territorial Epidemiologists
During week 18 the following influenza activity was reported:
- Local influenza activity was reported by five states (Maine, Maryland, Massachusetts, New Jersey, and South Carolina).
- Sporadic influenza activity was reported by the District of Columbia, Puerto Rico, and 30 states (Alabama, Alaska, Arizona, California, Colorado, Connecticut, Florida, Georgia, Hawaii, Illinois, Indiana, Louisiana, Michigan, Minnesota, Mississippi, Missouri, Montana, Nevada, New Hampshire, New York, North Dakota, Oregon, Pennsylvania, South Dakota, Tennessee, Texas, Vermont, Washington, Wisconsin, and Wyoming).
- No influenza activity was reported by 15 states (Arkansas, Delaware, Idaho, Iowa, Kansas, Kentucky, Nebraska, New Mexico, North Carolina, Ohio, Oklahoma, Rhode Island, Utah, Virginia, and West Virginia).
--------------------------------------------------------------------------------
A description of surveillance methods is available at: http://www.cy118119.com/flu/weekly/fluactivity.htm
- Page last updated May 9, 2008. Error processing SSI file