Error processing SSI file
Error processing SSI file
Weekly Report: Influenza Summary Update
Week ending April 21, 2007-Week 16
Error processing SSI fileSynopsis:
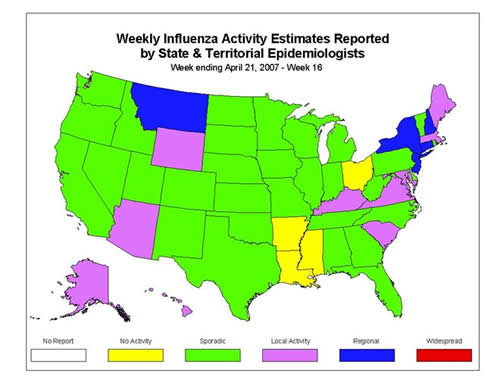
During week 16 (April 15 ?21, 2007)*, influenza activity continued to decrease in the United States. Data from the U.S. World Health Organization (WHO) and National Respiratory and Enteric Virus Surveillance System (NREVSS) collaborating laboratories indicated a decrease in the percentage of specimens testing positive for influenza. Other surveillance systems also indicated a decrease in influenza activity. The percentage of visits for ILI to sentinel providers decreased during week 16 and was below the national baseline for the fourth consecutive week. Five states reported regional influenza activity; 10 states reported local influenza activity; the District of Columbia, New York City, and 31 states reported sporadic influenza activity; and four states reported no influenza activity. The number of jurisdictions reporting widespread or regional influenza activity decreased from 11 for week 15 to five for week 16. The percent of deaths due to pneumonia and influenza remained below baseline level.
Laboratory Surveillance*:
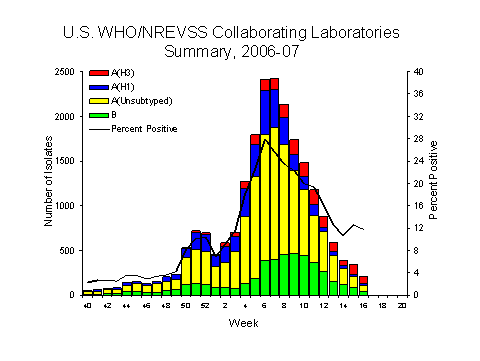
During week 16, WHO and NREVSS laboratories reported 1,821 specimens tested for influenza viruses, 215 (11.8%) of which were positive: 20 influenza A (H1) viruses, 87 influenza A (H3) viruses, 72 influenza A viruses that were not subtyped, and 36 influenza B viruses. Of the 87 influenza A (H3) viruses reported for week 16, 73 (83.9%) were reported from one state.
Since October 1, 2006, WHO and NREVSS laboratories have tested a total of 161,165 specimens for influenza viruses and 21,861 (13.5%) were positive. Among the 21,861 influenza viruses, 17,397 (79.6%) were influenza A viruses and 4,464 (20.4%) were influenza B viruses. Five thousand four hundred three (31.1%) of the 17,397 influenza A viruses have been subtyped: 3,709 (68.6%) were influenza A (H1) viruses and 1,694 (31.4%) were influenza A (H3) viruses. Among specimens tested for influenza during the most recent three weeks (March 25 ?April 14, 2007), on a regional basis, the percent of specimens testing positive for influenza were as follows:
April 1 – 21, 2007 (specimens testing positive) |
||
< 10% positive |
10-20% positive |
> 20% positive |
West North Central (5.0%) |
New England (13.3%) |
South Atlantic (25.3%) |
West South Central (3.9%) |
Mid Atlantic (14.6%) |
|
Mountain (7.0%) |
East North Central (11.2%) |
|
Pacific (5.7%) |
East South Central (11.8%) |
|
View Chart Data | View Full Screen
Composition of the 2006-07 Influenza Vaccine:
WHO has recommended that the 2007-08 trivalent influenza vaccine for the Northern Hemisphere contain A/Solomon Islands/3/2006-like (H1N1), A/Wisconsin/67/2005-like (H3N2), and B/Malaysia/2506/2004-like viruses. The influenza A (H1N1) component has been changed from the 2006-07 season vaccine components. A/Solomon Islands/3/2006 is a recent antigenic variant of the current vaccine strain A/New Caledonia/20/99. The influenza A (H3N2) and influenza B components remain the same. B/Ohio/1/2005 is antigenically equivalent to B/Malaysia/2506/2004. This recommendation was based on antigenic analyses of recently isolated influenza viruses, epidemiologic data, and post-vaccination serologic studies in humans.
Antigenic Characterization:
CDC has antigenically characterized 619 influenza viruses [324 influenza A (H1), 123 influenza A (H3) viruses, and 172 influenza B viruses] collected by U.S. laboratories since October 1, 2006.
Influenza A (H1) [324]Influenza A (H3) [123]?Three hundred and one (93%) of the 324 viruses characterized were similar to A/New Caledonia/20/99-like, which is the influenza A (H1) component of the 2006-07 influenza vaccine.
?Twenty-three (7%) of the 324 viruses showed somewhat reduced titers with antisera produced against A/New Caledonia/20/99 and are similar to A/Solomon Islands/3/2006-like. A/Solomon Islands/3/2006 is a recent antigenic variant of A/New Caledonia/20/99.
Influenza B (B/Victoria/02/87 and B/Yamagata/16/88 lineages) [172]?Thirty-five (28%) of the 123 viruses were characterized as A/Wisconsin/67/2005-like, which is the influenza A (H3) component of the 2006-07 influenza vaccine.
?Eighty-eight (72%) of the 123 viruses showed somewhat reduced titers with antisera produced against A/Wisconsin/67/2005.
Victoria lineage [128]
?One hundred and twenty-eight (74%) of the 172 influenza B viruses characterized belong to the B/Victoria lineage of viruses.Yamagata lineage [44]o Eighty-one (63%) of these 128 viruses were similar to B/Ohio/01/2005, the B component of the 2006-07 influenza vaccine.
o Forty-seven (37%) of these 128 viruses showed somewhat reduced titers with antisera produced against B/Ohio/01/2005.
?Forty-four (26%) of the 172 influenza B viruses characterized belong to the B/Yamagata lineage of viruses.
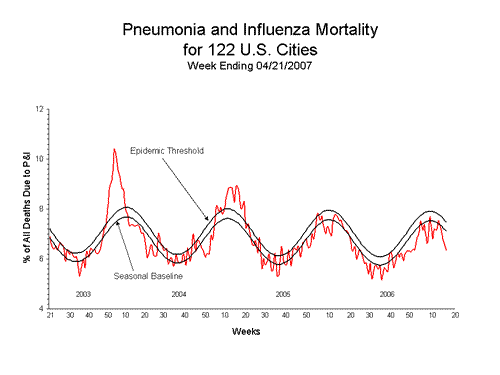
Pneumonia and Influenza (P&I) Mortality Surveillance*:
During week 16, 6.4% of all deaths were reported as due to pneumonia or influenza. This percentage is below the epidemic threshold of 7.5% for week 16.
Influenza-Associated Pediatric Mortality*:
Six influenza-associated pediatric deaths were reported during week 16. Since October 1, 2006, CDC has received 49 reports of influenza-associated pediatric deaths that occurred during the current season.
Influenza-Associated Pediatric Hospitalizations*:
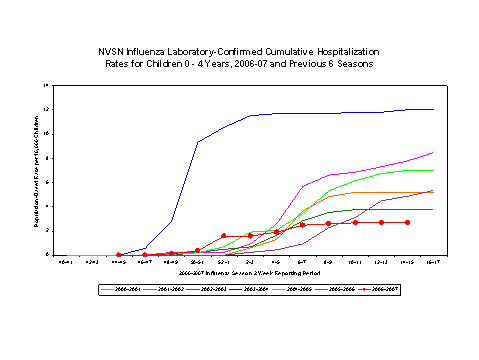
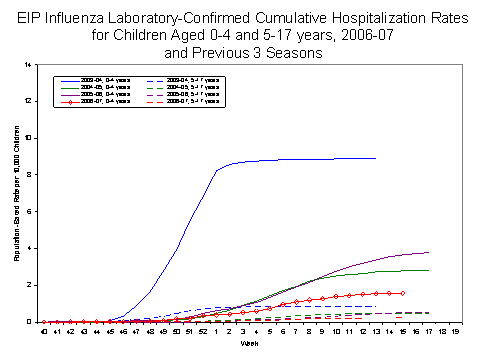
Laboratory-confirmed influenza-associated pediatric hospitalizations are monitored in two population-based surveillance networks?/sup>: the Emerging Infections Program (EIP) and the New Vaccine Surveillance Network (NVSN).
During November 5, 2006 to April 14, 2007, the preliminary laboratory-confirmed influenza-associated hospitalization rate for children 0-4 years old in the NVSN was 2.69 per 10,000.
During October 1, 2006 to April 14, 2007, the preliminary laboratory-confirmed influenza-associated hospitalization rate reported by the EIP for children 0?7 years old was 0.61 per 10,000. For children aged 0-4 years and 5-17 years, the rate was 1.58 per 10,000 and 0.24 per 10,000, respectively.
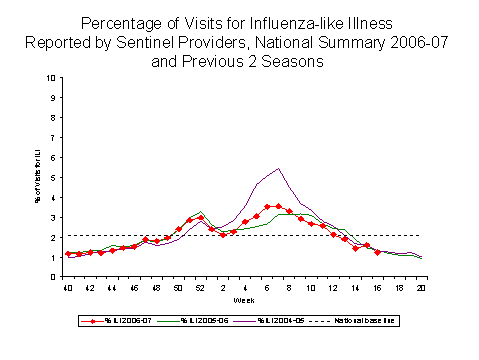
Influenza-like Illness Surveillance*:
During week 16, 1.2%** of patient visits to U.S. sentinel providers were due to ILI. This percentage is below the national baseline*** of 2.1%.
One out of nine surveillance regions**** reported ILI at their region-specific baseline***:
Region |
Reported ILI (%) |
Region-Specific Baseline (%) |
New England |
1.2 |
1.2 |
Mid Atlantic |
1.1 |
2.6 |
East North Central |
1.1 |
1.9 |
West North Central |
0.5 |
1.5 |
South Atlantic |
1.3 |
2.3 |
East South Central |
0.9 |
2.4 |
West South Central |
2.3 |
3.0 |
Mountain |
1.1 |
1.7 |
Pacific |
1.1 |
3.2 |
View
Chart Data
| View Full Screen
Influenza Activity as Assessed by State and Territorial Epidemiologists*:
During week 16, the following influenza activity唵 was reported:
?Regional activity was reported by five states (Connecticut, Montana, New Hampshire, New Jersey, and New York).
?Local activity was reported by ten states (Alaska, Arizona, Hawaii, Kentucky, Maine, Maryland, Massachusetts, South Carolina, Virginia, and Wyoming).
?Sporadic activity was reported by the District of Columbia, New York City, and 31 states (Alabama, California, Colorado, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kansas, Michigan, Minnesota, Missouri, Nebraska, Nevada, New Mexico, North Carolina, North Dakota, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Dakota, Tennessee, Texas, Utah, Vermont, Washington, West Virginia, and Wisconsin).
?Arkansas, Louisiana, Mississippi, and Ohio reported no influenza activity
--------------------------------------------------------------------------------
Report prepared April 27, 2007 Error processing SSI file