Error processing SSI file
Weekly Report: Influenza Summary Update
2008-2009 Influenza Season Week 26 ending July 4, 2009
All data are preliminary and may change as more reports are received.On June 11, the World Health Organization raised the pandemic alert level from Phase 5 to Phase 6 indicating that an influenza pandemic is underway.
Synopsis:
During week 26 (June 28-July 4, 2009), influenza activity decreased in the United States, however, there were still higher levels of influenza-like illness than is normal for this time of year.
- One thousand five hundred five (26.1%) specimens tested by U.S. World Health Organization (WHO) and National Respiratory and Enteric Virus Surveillance System (NREVSS) collaborating laboratories and reported to CDC/Influenza Division were positive for influenza.
- Over 97% of all subtyped influenza A viruses being reported to CDC were novel influenza A (H1N1) viruses.
- The proportion of deaths attributed to pneumonia and influenza (P&I) was equal to the epidemic threshold.
- Five influenza-associated pediatric deaths were reported and all five deaths were associated with novel influenza A (H1N1) virus infection.
- The proportion of outpatient visits for influenza-like illness (ILI) was below national and region-specific baseline levels.
- Nine states reported geographically widespread influenza activity, 12 states and Puerto Rico reported regional influenza activity, 10 states and the District of Columbia reported local influenza activity, 18 states reported sporadic influenza activity, and one state did not report.
HHS Surveillance Regions* |
Data for current week | Data cumulative for the season | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Out-patient ILI?/strong> | % positive for flu?/strong> | Number of jurisdictions reporting regional or widespread activity?/strong> | A (H1) | A (H3) | Novel A (H1N1) | A (unable to subtype)?/strong> | A (Sub-typing not performed) | B | Pediatric Deaths | |
| Nation | Normal | 26.1 % | 22 of 52 | 7,913 | 2,339 | 20,818 | 829 | 16,076 | 10,559 | 89 |
| Region I | Normal | 25.3 % | 4 of 6 | 521 | 151 | 1,719 | 14 | 1,644 | 800 | 3 |
| Region II | Normal | 34.7 % | 2 of 3 | 277 | 140 | 924 | 20 | 2,201 | 711 | 15 |
| Region III | Normal | 57.1 % | 4 of 6 | 1,335 | 219 | 3,324 | 11 | 826 | 1,360 | 9 |
| Region IV | Normal | 29.7 % | 4 of 8 | 831 | 130 | 790 | 47 | 2,349 | 1,220 | 6 |
| Region V | Normal | 44.7 % | 2 of 6 | 1,650 | 197 | 7,883 | 172 | 840 | 1,411 | 15 |
| Region VI | Normal | 25.2 % | 0 of 5 | 797 | 266 | 2,118 | 5 | 4,229 | 2,628 | 15 |
| Region VII | Normal | 35.1 % | 0 of 4 | 523 | 74 | 590 | 258 | 483 | 535 | 0 |
| Region VIII | Normal | 18.1 % | 1 of 6 | 530 | 217 | 1,063 | 59 | 1,569 | 499 | 7 |
| Region IX | Normal | 16.1 % | 4 of 4 | 1,061 | 632 | 864 | 34 | 1,455 | 704 | 17 |
| Region X | Normal | 22.8 % | 1 of 4 | 388 | 313 | 1,543 | 209 | 480 | 691 | 2 |
* HHS regions (Region I: CT, ME, MA, NH, RI, VT; Region II: NJ, NY, Puerto Rico, US Virgin Islands; Region III: DE, DC, MD, PA, VA, WV; Region IV: AL, FL, GA, KY, MS, NC, SC, TN; Region V: IL, IN, MI, MN, OH, WI; Region VI: AR, LA, NM, OK, TX; Region VII: IA, KS, MO, NE;
Region VIII: CO, MT, ND, SD, UT, WY; Region IX: AZ, CA, Guam, HI, NV; and Region X: AK, ID, OR, WA)
?Elevated means the % of visits for ILI is at or above the national or region-specific baseline
?National data are for current week; regional data are for the most recent three weeks
?Includes all 50 states, the District of Columbia, and Puerto Rico
?The majority of influenza A viruses that cannot be sub-typed as seasonal influenza viruses are novel A (H1N1) influenza viruses upon further testing
U.S. Virologic Surveillance:
WHO and NREVSS collaborating laboratories located in all 50 states and Washington D.C. report to CDC the number of respiratory specimens tested for influenza.
During the 2008-09 season, influenza A (H1), A (H3), and B viruses have co-circulated in the United States. On April 15 and 17, 2009, CDC confirmed the first two cases of novel influenza A (H1N1) virus in the United States. As of July 10, 2009, 37,246 confirmed and probable infections with novel influenza A (H1N1) virus and 211 deaths (6 deaths in individuals 0-4 years, 34 deaths in individuals 5-24 years, 87 deaths in adults 25-49 years, 50 deaths in adults 50-64 years, 19 deaths in adults age 65 and older and 15 deaths with unknown age) have been identified by CDC and state and local public health departments. Reporting of novel influenza A (H1N1) viruses by U.S. WHO collaborating laboratories began during week 17 (week ending May 2, 2009). The results of tests performed during the current week are summarized in the table below.
| Week 26 | |
|---|---|
| No. of specimens tested | 5,778 |
| No. of positive specimens (%) | 1,505 (26.1%) |
| Positive specimens by type/subtype | |
| Influenza A | 1,501 (99.7%) |
| A (novel H1N1) | 903 (60.2%) |
| 487 (32.4%) | |
| A (unable to subtype) | 88 (5.9%) |
| A (H3) | 4 (0.3%) |
| A (H1) | 19 (1.3%) |
| Influenza B | 4 (0.3%) |
During week 26, seasonal influenza A (H1), A (H3), and B viruses co-circulated at low levels with novel influenza A (H1N1) viruses. Over 97% of all subtyped influenza A viruses being reported to CDC this week were novel influenza A (H1N1) viruses.
The increase in the percentage of specimens testing positive for influenza by WHO and NREVSS collaborating laboratories may be due in part to changes in testing practices by health care providers, triaging of specimens by public health laboratories, an increase in the number of specimens collected from outbreaks, and other factors.
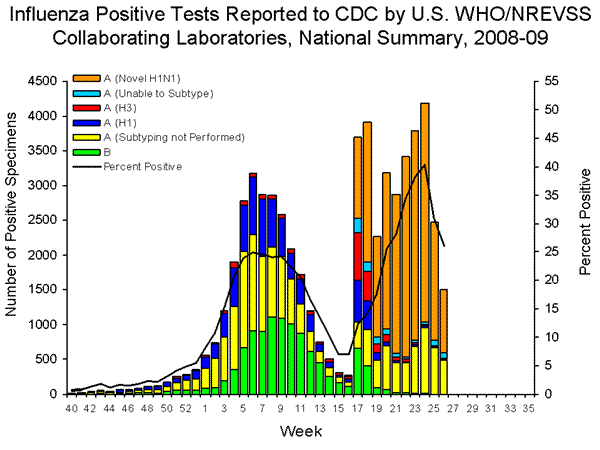
View WHO-NREVSS Regional Bar Charts| View Chart Data | View Full Screen
Antigenic Characterization:
CDC has antigenically characterized 1,729 seasonal human influenza viruses [995 influenza A (H1), 171 influenza A (H3) and 563 influenza B viruses] collected by U.S. laboratories since October 1, 2008, and 233 novel influenza A (H1N1) viruses.
All 995 influenza seasonal A (H1) viruses are related to the influenza A (H1N1) component of the 2008-09 influenza vaccine (A/Brisbane/59/2007). All 171 influenza A (H3N2) viruses are related to the A (H3N2) vaccine component (A/Brisbane/10/2007).
All 233 novel influenza A (H1N1) viruses are related to the A/California/07/2009 (H1N1) reference virus selected by WHO as a potential candidate for novel influenza A (H1N1) vaccine.
Influenza B viruses currently circulating can be divided into two distinct lineages represented by the B/Yamagata/16/88 and B/Victoria/02/87 viruses. Sixty-seven influenza B viruses tested belong to the B/Yamagata lineage and are related to the vaccine strain (B/Florida/04/2006). The remaining 496 viruses belong to the B/Victoria lineage and are not related to the vaccine strain.
Data on antigenic characterization should be interpreted with caution given that antigenic characterization data is based on hemagglutination inhibition (HI) testing using a panel of reference ferret antisera and results may not correlate with clinical protection against circulating viruses provided by influenza vaccination.
Annual influenza vaccination is expected to provide the best protection against those virus strains that are related to the vaccine strains, but limited to no protection may be expected when the vaccine and circulating virus strains are so different as to be from different lineages, as is seen with the two lineages of influenza B viruses. Antigenic characterization of novel influenza A (H1N1) viruses indicates that these viruses are antigenically and genetically unrelated to seasonal influenza A (H1N1) viruses, suggesting that little to no protection would be expected from vaccination with seasonal influenza vaccine.
Antiviral Resistance:
Since October 1, 2008, 1,066 seasonal influenza A (H1N1), 198 influenza A (H3N2), and 585 influenza B viruses have been tested for resistance to the neuraminidase inhibitors (oseltamivir and zanamivir). Also, 1,068 seasonal influenza A (H1N1) and 206 influenza A (H3N2) viruses have been tested for resistance to the adamantanes (amantadine and rimantadine). Two hundred sixty-four novel influenza A (H1N1) viruses have been tested for resistance to the neuraminidase inhibitors (oseltamivir and zanamivir). Two hundred forty-two novel influenza A (H1N1) viruses have been tested for resistance to the adamantanes (amantadine and rimantadine). The results of antiviral resistance testing performed on these viruses are summarized in the table below.
| Isolates tested (n) | Resistant Viruses, Number (%) |
Isolates tested (n) | Resistant Viruses, Number (%) | ||
|---|---|---|---|---|---|
| Oseltamivir | Zanamivir | Adamantanes | |||
| Seasonal Influenza A (H1N1) | 1,066 | 1,061 (99.5%) | 0 (0) | 1,068 | 6 (0.6%) |
| Influenza A (H3N2) | 198 | 0 (0) | 0 (0) | 206 | 206 (100%) |
| Influenza B | 585 | 0 (0) | 0 (0) | N/A* | N/A* |
| Novel Influenza A (H1N1) | 264 | 0 (0) | 0 (0) | 242 | 242 (100%) |
The novel influenza A (H1N1) virus is susceptible to both neuraminidase inhibitor antiviral medications zanamivir and oseltamivir. It is resistant to the adamantane antiviral medications, amantadine and rimantadine. Antiviral treatment with either oseltamivir or zanamivir is recommended for all patients with confirmed, probable or suspected cases of novel influenza A (H1N1) virus infection who are hospitalized or who are at higher risk for seasonal influenza complications.
Three cases of oseltamivir resistant novel influenza A (H1N1) viruses have been detected worldwide, none of which were detected in the U.S. However, one of the three viruses was detected in a specimen collected from a child that became ill in California and subsequently traveled to Hong Kong where the respiratory specimen was collected, and the oseltamivir resistant novel H1N1 strain was detected. Enhanced antiviral resistance testing in California has not revealed any other oseltamivir resistant novel influenza A (H1N1) viruses. The other two cases of oseltamivir resistant novel H1N1 viruses were detected in Japan and Denmark (http://www.who.int/csr/disease/swineflu/newsbriefs/h1n1_antiviral_resistance_20090708/en/index.html). Additional information on antiviral recommendations for treatment and chemoprophylaxis of novel influenza A (H1N1) infection is available at http://www.cy118119.com/h1n1flu/recommendations.htm
Three seasonal influenza A (H1N1) viruses collected between February 8 and May 11, 2009 were found to be resistant to both oseltamivir and the adamantanes (amantadine and rimantadine). All influenza A (H1N1) viruses tested retain their sensitivity to zanamivir. The three dually resistant viruses represent less than 0.5% of all seasonal influenza A (H1N1) viruses tested during the 2008-09 influenza season, and as a result, no changes to the influenza antiviral treatment or prophylaxis recommendations will be made at this time. CDC will continue to monitor trends in antiviral resistance over the summer and throughout the upcoming 2009-10 influenza season.
Pneumonia and Influenza (P&I) Mortality Surveillance
During week 26, 6.6% of all deaths reported through the 122-Cities Mortality Reporting System were due to P&I. This percentage was equal to the epidemic threshold of 6.6% for week 26.
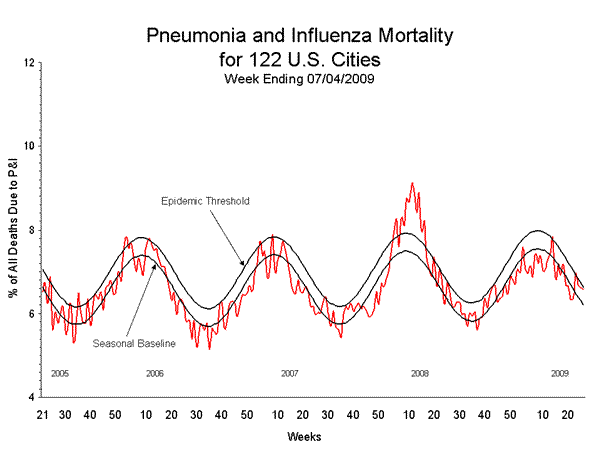
View Full Screen
Influenza-Associated Pediatric Mortality
Five influenza-associated pediatric deaths were reported to CDC during week 26 (Arizona, New Jersey, New York, and New York City [2]). All five deaths were associated with novel influenza A (H1N1) virus infection. The deaths reported this week occurred during weeks 22-26 (the weeks ending June 6 ?July 4, 2009). Since September 28, 2008, CDC has received 89 reports of influenza-associated pediatric deaths that occurred during the current influenza season, 22 of which were due to novel influenza A (H1N1) virus infections.
Of the 39 children who had specimens collected for bacterial culture from normally sterile sites, 15 (38.5%) were positive; Staphylococcus aureus was identified in nine (60.0%) of the 15 children. Four of the S. aureus isolates were sensitive to methicillin and five were methicillin resistant. Thirteen (86.7%) of the 15 children with bacterial coinfections were five years of age or older and 10 (66.7%) of the 15 children were 12 years of age or older. Seven of the 22 children with confirmed novel influenza A (H1N1) infection had a specimen collected from a normally sterile site; one of the seven children had a positive bacterial culture. An increase in the number of influenza-associated pediatric deaths with bacterial coinfections was first recognized during the 2006-07 influenza season. In January 2008, interim testing and reporting recommendations were released regarding influenza and bacterial coinfections in children and are available at (http://www2a.cdc.gov/HAN/ArchiveSys/ViewMsgV.asp?AlertNum=00268).
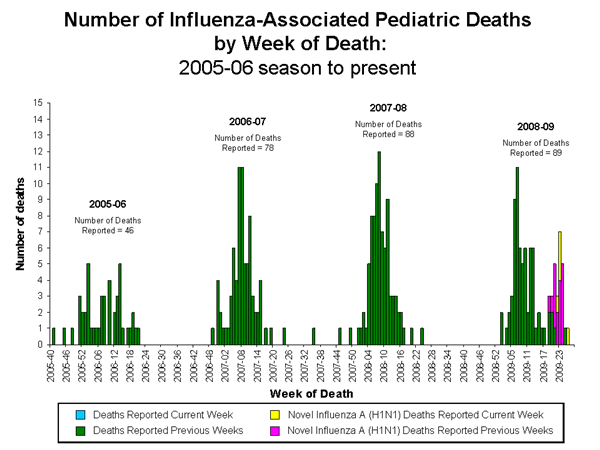
View Full Screen
Influenza-Associated Hospitalizations
Laboratory-confirmed influenza-associated hospitalizations are monitored in two population-based surveillance networks: the New Vaccine Surveillance Network (NVSN) and the Emerging Infections Program (EIP).
During October 12, 2008 to June 27, 2009, the preliminary laboratory-confirmed influenza-associated hospitalization rate for children 0-4 years old in the NVSN was 4.42 per 10,000. Because of case identification methods utilized in this study, there is a delay from the date of hospitalization to the date of report.
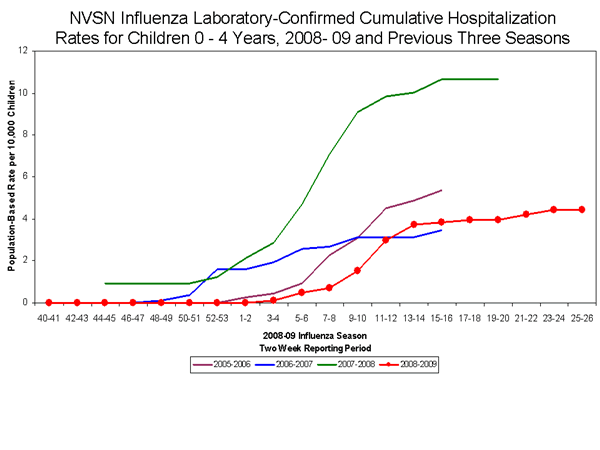
View Full Screen
During April 15, 2009 to July 4, 2009, the following preliminary laboratory-confirmed overall influenza associated hospitalization rates were reported by the EIP (rates include type A, type B, and novel H1N1):
Rates for children aged 0-23 months, 2-4 years, and 5-17 years were 1.6, 0.6, and 0.5 per 10,000, respectively. Rates for adults aged 18-49 years, 50-64 years, and >= 65 years were 0.2, 0.2, and 0.3 per 10,000, respectively.
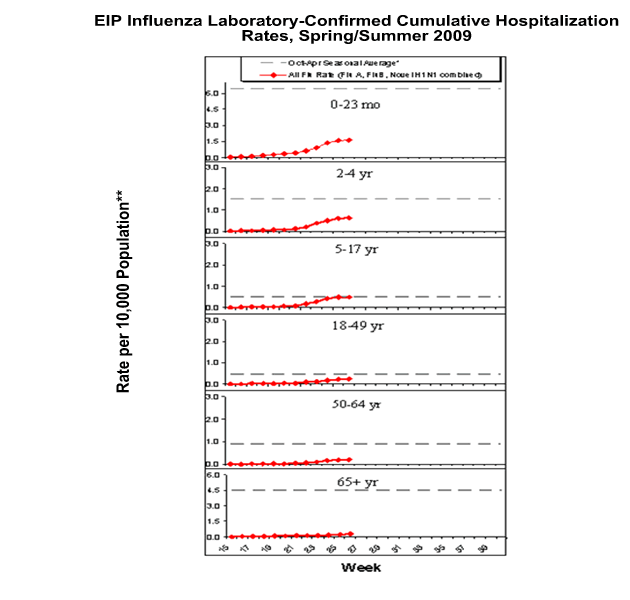
*This value represents an age group-specific average influenza rate from October 1 to April 30 from the 2005-06, 2006-07, and 2007-08 influenza seasons.
**Note: The scales for the 0-23 month and the >= 65 years age groups differ from other age groups.
View Full Screen
Outpatient Illness Surveillance:
Nationwide during week 26, 1.5% of patient visits reported through the U.S. Outpatient Influenza-like Illness Surveillance Network (ILINet) were due to influenza-like illness (ILI). This percentage is below the national baseline of 2.4%.
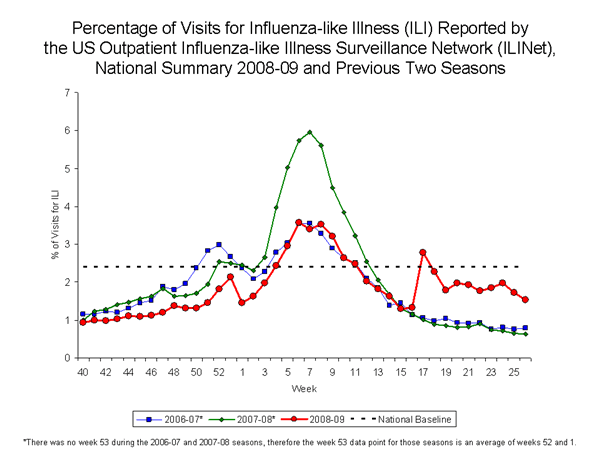
View ILINet Regional Charts | View Chart Data |View Full Screen
On a regional level, the percentage of outpatient visits for ILI ranged from 0.3% to 3.2%. All 10 regions reported percentages of visits for ILI below their respective region-specific baselines. ILI decreased during week 26 in seven of 10 regions compared to week 25.
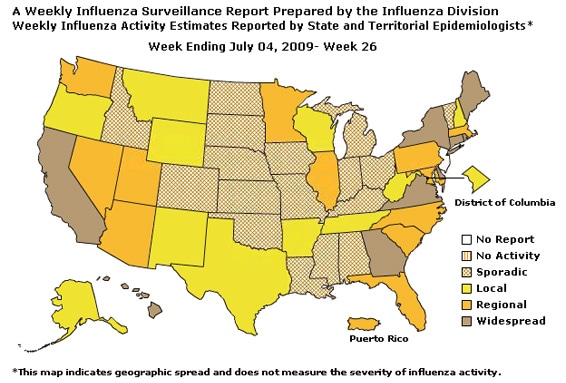
Geographic Spread of Influenza as Assessed by State and Territorial Epidemiologists:
The influenza activity reported by state and territorial epidemiologists indicates geographic spread of both seasonal influenza and novel influenza A (H1N1) viruses and does not measure the severity of influenza activity.
During week 26, the following influenza activity was reported:
- Widespread influenza activity was reported by nine states (California, Connecticut, Delaware, Georgia, Hawaii, Maine, New York, Rhode Island, and Virginia).
- Regional influenza activity was reported by Puerto Rico and 12 states (Arizona, Florida, Illinois, Maryland, Massachusetts, Minnesota, Nevada, North Carolina, Pennsylvania, South Carolina, Utah, and Washington).
- Local influenza activity was reported by the District of Columbia and 10 states (Alaska, Arkansas, New Hampshire, New Mexico, Oregon, Tennessee, Texas, West Virginia, Wisconsin, and Wyoming).
- Sporadic activity was reported by 18 states (Alabama, Colorado, Idaho, Indiana, Iowa, Kansas, Kentucky, Louisiana, Michigan, Mississippi, Missouri, Montana, Nebraska, North Dakota, Ohio, Oklahoma, South Dakota, and Vermont).
- One state did not report (New Jersey).
--------------------------------------------------------------------------------
A description of surveillance methods is available at:
http://www.cy118119.com/flu/weekly/fluactivity.htm
- Page last updated July 10, 2009.