Error processing SSI file
Error processing SSI file
Weekly Report: Influenza Summary Update
Week ending April 28, 2007-Week 17
Error processing SSI fileSynopsis:
During week 17 (April 22 ?28, 2007)*, influenza activity continued to decrease in the United States. Data from the U.S. World Health Organization (WHO) and National Respiratory and Enteric Virus Surveillance System (NREVSS) collaborating laboratories indicated a decrease in the percentage of specimens testing positive for influenza. The percentage of visits for ILI to sentinel providers decreased during week 17 and was below the national baseline for the sixth consecutive week. Three states reported regional influenza activity; nine states reported local influenza activity; the District of Columbia, New York City, and 30 states reported sporadic influenza activity; and eight states reported no influenza activity. The number of jurisdictions reporting widespread or regional influenza activity decreased from five for week 16 to three for week 17. The percent of deaths due to pneumonia and influenza remained below baseline levels for the entire influenza season to date.
Laboratory Surveillance*:
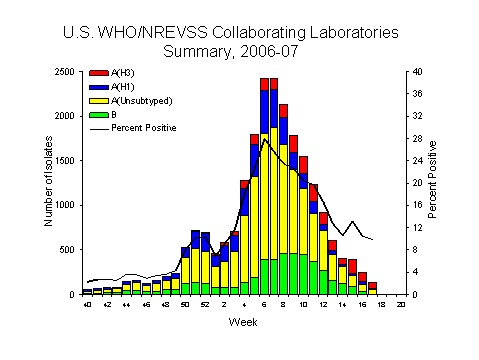
During week 17, WHO and NREVSS laboratories reported 1,673 specimens tested for influenza viruses, 164 (9.8%) of which were positive: 15 influenza A (H1) viruses, 71 influenza A (H3) viruses, 50 influenza A viruses that were not subtyped, and 28 influenza B viruses.
Since October 1, 2006, WHO and NREVSS laboratories have tested a total of 164,866 specimens for influenza viruses and 22,454 (13.6%) were positive. Among the 22,454 influenza viruses, 17,856 (79.5%) were influenza A viruses and 4,598 (20.5%) were influenza B viruses. Five thousand seven hundred seventy (32.3%) of the 17,856 influenza A viruses have been subtyped: 3,813 (66.1%) were influenza A (H1) viruses and 1,957 (33.9%) were influenza A (H3) viruses. Among specimens tested for influenza during the most recent three weeks (April 8 ?28, 2007), on a regional basis, the percent of specimens testing positive for influenza were as follows:
April 8 – 28, 2007 (specimens testing positive) |
||
< 10% positive |
10-20% positive |
> 20% positive |
East North Central (8.7%) |
New England (10.1%) |
South Atlantic (27.9%) |
West North Central (2.9%) |
Mid Atlantic (13.1%) |
|
East South Central (3.7%) |
Pacific (12.1%) |
|
West South Central (3.6%) |
|
|
Mountain (6.2%) |
|
|
View
Chart Data |
View
Full Screen
Composition of the 2006-07 Influenza Vaccine:
WHO has recommended that the 2007-08 trivalent influenza vaccine for the Northern Hemisphere contain A/Solomon Islands/3/2006-like (H1N1), A/Wisconsin/67/2005-like (H3N2), and B/Malaysia/2506/2004-like viruses. The influenza A (H1N1) component has been changed from the 2006-07 season vaccine components. A/Solomon Islands/3/2006 is a recent antigenic variant of the current vaccine strain A/New Caledonia/20/99. The influenza A (H3N2) and influenza B components remain the same. B/Ohio/1/2005 is antigenically equivalent to B/Malaysia/2506/2004. This recommendation was based on antigenic analyses of recently isolated influenza viruses, epidemiologic data, and post-vaccination serologic studies in humans.
Antigenic Characterization:
CDC has antigenically characterized 647 influenza viruses [325 influenza A (H1), 145 influenza A (H3) viruses, and 177 influenza B viruses] collected by U.S. laboratories since October 1, 2006.
Influenza A (H1) [325]Influenza A (H3) [145]?Three hundred and two (93%) of the 325 viruses characterized were similar to A/New Caledonia/20/99-like, which is the influenza A (H1) component of the 2006-07 influenza vaccine.
?Twenty-three (7%) of the 325 viruses showed somewhat reduced titers with antisera produced against A/New Caledonia/20/99 and are similar to A/Solomon Islands/3/2006-like. A/Solomon Islands/3/2006 is a recent antigenic variant of A/New Caledonia/20/99.
Influenza B (B/Victoria/02/87 and B/Yamagata/16/88 lineages) [177]?Forty (28%) of the 145 viruses were characterized as A/Wisconsin/67/2005-like, which is the influenza A (H3) component of the 2006-07 influenza vaccine.
?One hundred and five (72%) of the 145 viruses showed somewhat reduced titers with antisera produced against A/Wisconsin/67/2005.
Victoria lineage [133]
?One hundred and thirty-three (75%) of the 177 influenza B viruses characterized belong to the B/Victoria lineage of viruses.Yamagata lineage [44]o Eighty-one (61%) of these 133 viruses were similar to B/Ohio/01/2005, the B component of the 2006-07 influenza vaccine.
o Fifty-two (39%) of these 133 viruses showed somewhat reduced titers with antisera produced against B/Ohio/01/2005.
?Forty-four (25%) of the 177 influenza B viruses characterized belong to the B/Yamagata lineage of viruses.
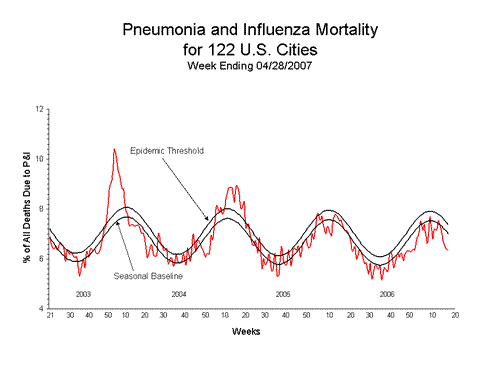
Pneumonia and Influenza (P&I) Mortality Surveillance*:
During week 17, 6.4% of all deaths were reported as due to pneumonia or influenza. This percentage is below the epidemic threshold of 7.4% for week 17.
Influenza-Associated Pediatric Mortality*:
Four influenza-associated pediatric deaths were reported during week 17. Since October 1, 2006, CDC has received 53 reports of influenza-associated pediatric deaths that occurred during the current season.
Influenza-Associated Pediatric Hospitalizations*:
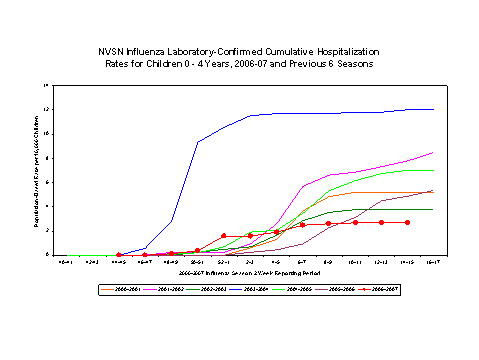
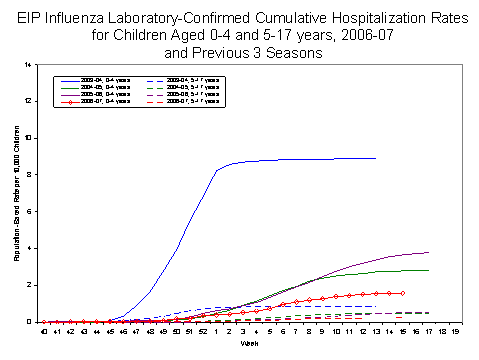
Laboratory-confirmed influenza-associated pediatric hospitalizations are monitored in two population-based surveillance networks?/sup>: the Emerging Infections Program (EIP) and the New Vaccine Surveillance Network (NVSN).
During November 5, 2006 to April 14, 2007, the preliminary laboratory-confirmed influenza-associated hospitalization rate for children 0-4 years old in the NVSN was 2.69 per 10,000.
During October 1, 2006 to April 14, 2007, the preliminary laboratory-confirmed influenza-associated hospitalization rate reported by the EIP for children 0?7 years old was 0.61 per 10,000. For children aged 0-4 years and 5-17 years, the rate was 1.58 per 10,000 and 0.24 per 10,000, respectively.
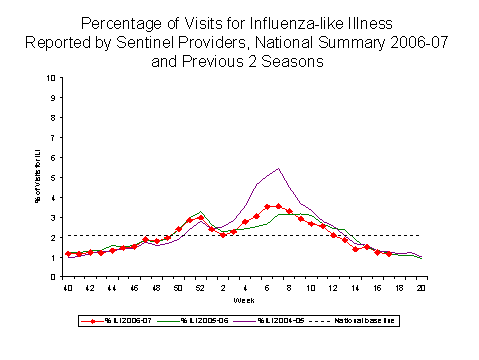
Influenza-like Illness Surveillance*:
During week 17, 1.2%** of patient visits to U.S. sentinel providers were due to ILI. This percentage is below the national baseline*** of 2.1%. All nine surveillance regions**** reported ILI below their region-specific baseline***:
View
Chart Data
| View Full Screen
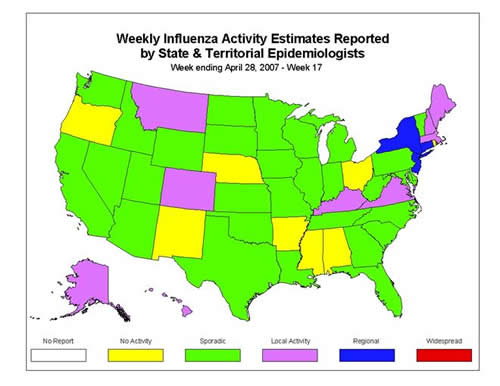
Influenza Activity as Assessed by State and Territorial Epidemiologists*:
During week 17, the following influenza activity唵 was reported:
?Regional activity was reported by three states (Connecticut, New Jersey, and New York).
?Local activity was reported by nine states (Alaska, Colorado, Hawaii, Kentucky, Maine, Massachusetts, Montana, New Hampshire, and Virginia).
?Sporadic activity was reported by the District of Columbia, New York City, and 30 states (Arizona, California, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kansas, Louisiana, Maryland, Michigan, Minnesota, Missouri, Nevada, North Carolina, North Dakota, Oklahoma, Pennsylvania, South Carolina, South Dakota, Tennessee, Texas, Utah, Vermont, Washington, West Virginia, Wisconsin, and Wyoming).
?Alabama, Arkansas, Mississippi, Nebraska, New Mexico, Ohio, Oregon, and Rhode Island reported no influenza activity
--------------------------------------------------------------------------------
Report prepared May 4, 2007 Error processing SSI file