Error processing SSI file
Error processing SSI file
Weekly Report: Influenza Summary Update
Week ending December 21, 2002-Week 51
Error processing SSI fileThe following information may be quoted:
Synopsis:
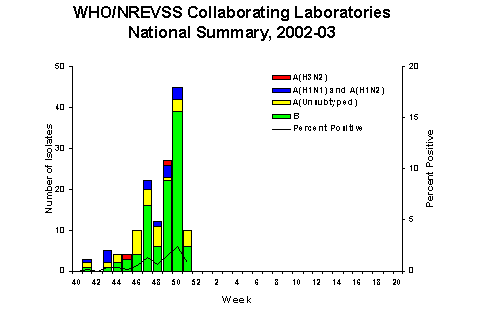
During week 51(December 15-21, 2002)*, 10 (0.8%) of the specimens tested by World Health Organization (WHO) and National Respiratory and Enteric Virus Surveillance System (NREVSS) laboratories were positive for influenza. The proportion of patient visits to sentinel providers for influenza-like illness (ILI) overall was 1.6%, which is less than the national baseline of 1.9%. The proportion of deaths attributed to pneumonia and influenza was 7.0%. One state and territorial health department reported widespread influenza activity, 5 reported regional activity, 22 reported sporadic activity, and 19 reported no influenza activity**.
Laboratory Surveillance*:
During week 51, WHO and NREVSS
laboratories reported 1,301 specimens tested for influenza
viruses, of which 10 (0.8%) were positive. Four unsubtyped
influenza A viruses and 6 influenza B viruses were identified.
Since September 29, WHO and NREVSS laboratories have tested
a total of 19,817 specimens for influenza viruses and 142
(0.7%) were positive. Of the 142 viruses identified, 42 (30%)
were influenza A viruses and 100 (70%) were influenza B viruses.
Fifteen (36%) of the 42 influenza A viruses have been subtyped;
13 were influenza A (H1)?viruses and 2 were influenza A (H3N2) viruses. Nineteen
states have reported laboratory-confirmed influenza. Influenza A viruses have
been identified in Florida, Hawaii, Louisiana, Massachusetts, Nebraska, New
Jersey, New York, North Carolina, Oregon, South Carolina, Texas, Virginia,
Washington, and Wisconsin. Influenza B viruses have been identified in Arkansas,
Arizona, Indiana, Louisiana, Nebraska, Nevada, New York, North Carolina, Oklahoma,
South Carolina, and Texas. Of the 142 viruses reported, 87 (61%) were identified
in the West South Central region*** and 35 (25%) were identified in the South
Atlantic region***.
Antigenic Characterization of Viral Isolates:
CDC has antigenically characterized 24 influenza viruses submitted by U.S. laboratories since September 29: seventeen influenza B viruses, two influenza A (H3N2) viruses, and 5 influenza A (H1)?viruses. Four of the influenza A (H1) viruses contained the N1 neuraminidase and one contained the N2 neuraminidase. The influenza B viruses, the A (H3N2) viruses, and the hemagglutinin protein of the A (H1) viruses were similar antigenically to the corresponding vaccine strains B/Hong Kong/330/01, A/Panama/2007/99 (H3N2), and A/New Caledonia/20/99 (H1N1), respectively.
Click here for more information about influenza A (H1N2) viruses
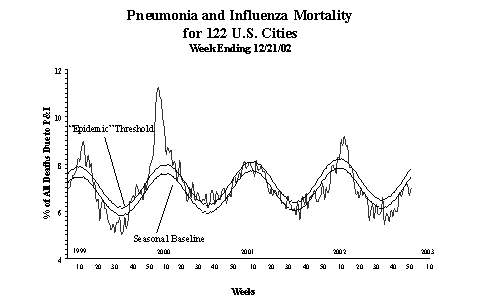
Pneumonia and Influenza (P&I) Mortality Surveillance:
During
week 51, the percentage of all deaths due to pneumonia
and influenza as reported by the vital statistics offices
of 122 U.S. cities was 7.0%. This percentage is below the
epidemic threshold of 7.8% for week 51.
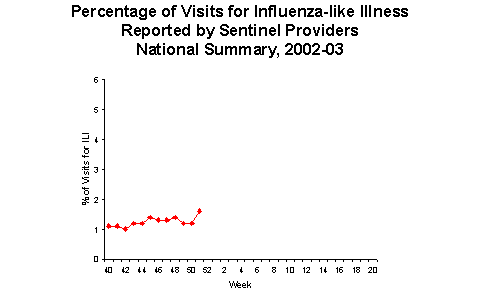
Influenza-like Illness Surveillance *:
During week 51, 1.6% of patient visits to U.S. sentinel providers were due to ILI. This percentage is less than the national baseline of 1.9%. On a regional level***, the percentage of visits for ILI ranged from 0.6% to 4.8%. Due to wide variability in regional level data, it is not appropriate to apply the national baseline to regional level data.
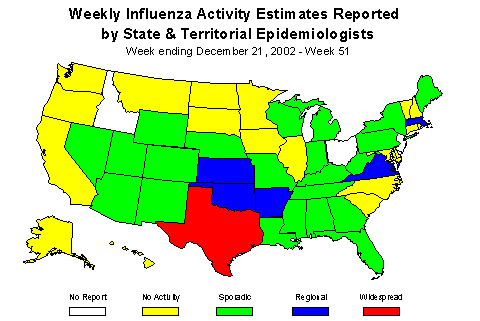
Influenza Activity as Assessed by State and Territorial Epidemiologists**:
Influenza activity was reported as widespread in Texas and regional in 5 states (Arkansas, Kansas, Massachusetts, Oklahoma, and Virginia). Twenty-two states, New York City, and Washington D.C reported sporadic activity, 19 states reported no influenza activity, and 3 states did not report.
* Reporting is incomplete for this week. Numbers may change
as more reports are received.
** Influenza activity is defined as influenza-like illness and/or culture-confirmed
influenza.
咺ncludes both the A (H1N1) and A (H1N2) influenza virus subtypes. The influenza
A (H1N2) strain appears to have resulted from the reassortment of the genes of
currently circulating influenza A (H1N1) and A (H3N2) subtypes. Because the hemagglutinin
proteins of the A (H1N2) viruses are similar to those of the currently circulating
A (H1N1) viruses and the neuraminidase proteins are similar to those of the currently
circulating A (H3N2) viruses, the 2002-03 influenza vaccine should provide protection
against A (H1N2) viruses.
*** Surveillance Regions: New England (Connecticut, Maine,
Massachusetts, New Hampshire, Vermont, Rhode Island); Mid-Atlantic (New
Jersey, New York City, Pennsylvania, Upstate New York); East North
Central (Illinois, Indiana, Michigan, Ohio, Wisconsin); West
North Central (Iowa, Kansas, Minnesota, Missouri, Nebraska, North
Dakota, South Dakota); South Atlantic (Delaware, Florida,
Georgia, Maryland, North Carolina, South Carolina, Virginia, Washington, D.C.,
West Virginia); East South Central (Alabama, Kentucky, Mississippi,
Tennessee); West South Central (Arkansas, Louisiana, Oklahoma,
Texas); Mountain (Arizona, Colorado, Idaho, Montana, Nevada,
New Mexico, Utah, Wyoming); Pacific (Alaska, California, Hawaii,
Oregon, Washington)
Report prepared December 30, 2002
Error processing SSI file