U.S. Virologic Surveillance
Clinical Laboratories
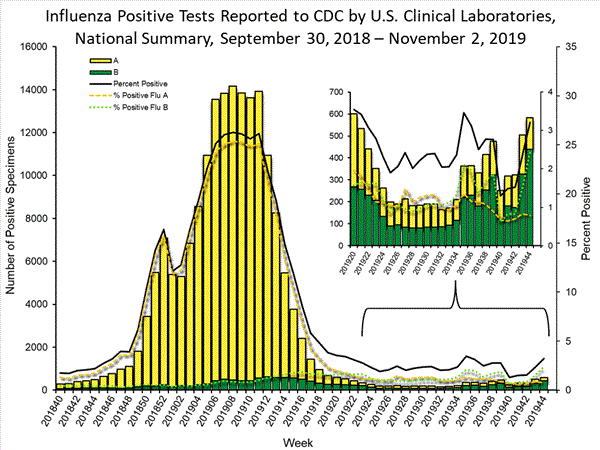
The results of tests performed by clinical laboratories nationwide are summarized below. Data from clinical laboratories (the percentage of specimens tested that are positive for influenza) are used to monitor whether influenza activity is increasing or decreasing.
| Week 44 | Data Cumulative since September 29, 2019 (week 40) |
|
|---|---|---|
| No. of specimens tested* | 18,126 | 101,524 |
| No. of positive specimens (%) | 582 (3.2%) | 1,978 (1.9%) |
| Positive specimens by type | ||
| Influenza A | 143 (24.6%) | 738 (37.3%) |
| Influenza B | 439 (75.4%) | 1,240 (62.7%) |
* The total number of specimens tested by clinical laboratories decreased this week for this season because one high volume laboratory has been removed due to questions about testing practices and data interpretation.
The majority (63%) of all influenza viruses and 75% of the influenza B viruses reported by clinical laboratories thus far this season were from the south and southeast regions (regions 4 and 6).
View Chart Data | View Full Screen
Public Health Laboratories
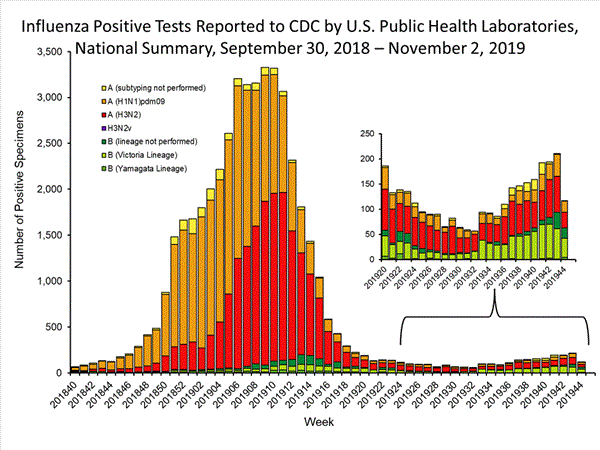
The results of tests performed by public health laboratories nationwide are summarized below. Data from public health laboratories are used to monitor the proportion of circulating viruses that belong to each influenza subtype/lineage.
| Week 44 | Data Cumulative since September 29, 2019 (week 40) |
|
|---|---|---|
| No. of specimens tested | 766 | 5,022 |
| No. of positive specimens | 117 | 873 |
| Positive specimens by type/subtype | ||
| Influenza A | 54 (46.2%) | 491 (56.2%) |
| (H1N1)pdm09 | 22 (41.5%) | 153 (34.8%) |
| H3N2 | 31 (58.5%) | 287 (65.2%) |
| Subtyping not performed | 1 | 51 |
| Influenza B | 63 (53.8%) | 382 (43.8%) |
| Yamagata lineage | 4 (9.5%) | 11 (3.7%) |
| Victoria lineage | 38 (90.5%) | 287 (96.3%) |
| Lineage not performed | 21 | 84 |
Nationally, influenza A(H3N2) and B/Victoria viruses have been reported more frequently than other influenza viruses this season; however, influenza A(H1N1)pdm09 viruses are also circulating widely. The predominant virus varies by region. For regional and state level data about circulating influenza viruses, please visit FluView Interactive.
View Chart Data | View Full Screen
For additional virologic surveillance information for this season and past seasons:
Surveillance Methods | FluView Interactive: National, Regional, and State Data or Age Data
Influenza Virus Characterization
CDC performs genetic and antigenic characterization of U.S. viruses submitted from state and local health laboratories using Right Size Roadmap submission guidance. These data are used to compare how similar the currently circulating influenza viruses are to the reference viruses used for developing new influenza vaccines and to monitor evolutionary changes that continually occur in influenza viruses circulating in humans. CDC also tests susceptibility of influenza viruses to antiviral medications including the neuraminidase inhibitors (oseltamivir, zanamivir, and peramivir) and the PA endonuclease inhibitor baloxavir.
Virus characterization data will be updated starting later this season when sufficient numbers of specimens have been tested.
Outpatient Illness Surveillance
ILINet
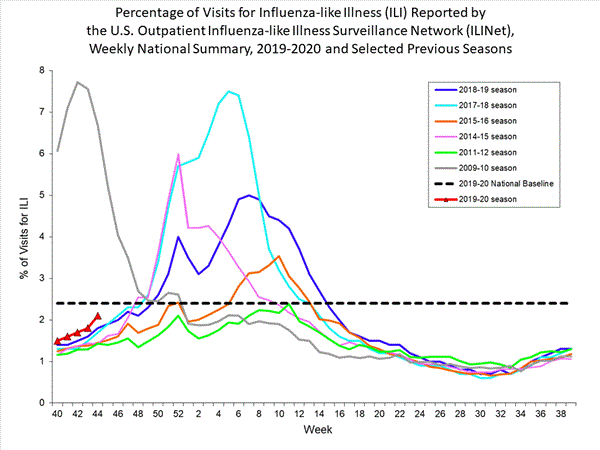
Nationwide during week 44, 2.1% of patient visits reported through the U.S. Outpatient Influenza-like Illness Surveillance Network (ILINet) were due to influenza-like illness (ILI). This percentage is below the national baseline of 2.4%.
View Chart Data | View Full Screen
On a regional level, the percentage of outpatient visits for ILI ranged from 1.2% to 3.8% during week 44. Region 6 (Arkansas, Louisiana, New Mexico, Oklahoma, and Texas) and Region 7 (Iowa, Kansas, Missouri, and Nebraska) reported a percentage of outpatient visits for ILI which is equal to their region-specific baselines. All other regions remained below their region-specific baselines.
ILI Activity Map
Data collected in ILINet are used to produce a measure of ILI activity* by state.
During week 44, the following ILI activity levels were experienced:
- High 鈥?Puerto Rico and one state (Louisiana) (Louisiana)
- Low 鈥?nine states (Alabama, Arkansas, Colorado, Georgia, Hawaii, Missouri, South Carolina, Texas, and Virginia)
- Minimal 鈥?the U.S. Virgin Islands, the District of Columbia, New York City, and 40 states (Alaska, Arizona, California, Connecticut, Delaware, Florida, Idaho, Illinois, Indiana, Iowa, Kansas, Kentucky, Maine, Maryland, Massachusetts, Michigan, Minnesota, Mississippi, Montana, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Dakota, Tennessee, Utah, Vermont, Washington, West Virginia, Wisconsin, and Wyoming)
*Data collected in ILINet may disproportionally represent certain populations within a state, and therefore, may not accurately depict the full picture of influenza activity for the whole state. Differences in the data presented here by CDC and independently by some state health departments likely represent differing levels of data completeness with data presented by the state likely being the more complete.
Additional information about medically attended visits for ILI this season and past seasons:
Surveillance Methods | FluView Interactive: National, Regional, and State Data or ILI Activity Map
Geographic Spread of Influenza as Assessed by State and Territorial Epidemiologists
The influenza activity reported by state and territorial epidemiologists indicates geographic spread of influenza viruses but does not measure the severity of influenza activity.
During week 44 the following influenza activity was reported:
- Widespread 鈥?two states (Louisiana and Maryland)
- Regional 鈥?one state (Alabama)
- Local 鈥?Puerto Rico and 15 states (Arizona, California, Florida, Georgia, Illinois, Indiana, Kentucky, Massachusetts, Mississippi, Nevada, New Hampshire, South Carolina, Tennessee, Texas and West Virginia).
- Sporadic 鈥?the District of Columbia, the U.S. Virgin Islands and 31 states (Alaska, Arkansas, Colorado, Connecticut, Delaware, Hawaii, Idaho, Iowa, Kansas, Maine, Michigan, Minnesota, Missouri, Montana, Nebraska, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, South Dakota, Utah, Vermont, Virginia, Washington, Wisconsin, and Wyoming).
- No activity 鈥?one state (Rhode Island)
- Guam did not report.
Additional information about the geographic spread of influenza this season and past seasons:
Surveillance Methods | FluView Interactive
Influenza-Associated Hospitalizations
The Influenza Hospitalization Surveillance Network (FluSurv-NET) conducts population-based surveillance for laboratory-confirmed influenza-related hospitalizations in select counties in the Emerging Infections Program (EIP) states and Influenza Hospitalization Surveillance Project (IHSP) states. FluSurv-NET estimated hospitalization rates will be updated weekly starting later this season when a sufficient number of hospitalizations have been reported. 聽
Additional FluSurv-NET data can be found at: http://gis.cdc.gov/GRASP/Fluview/FluHospRates.html and http://gis.cdc.gov/grasp/fluview/FluHospChars.html.
Pneumonia and Influenza (P&I) Mortality Surveillance
Due to technical issues, the National Center for Health Statistics (NCHS) mortality surveillance data for the week ending October 26, 2019 (week 43) will not be published this week. Reporting of this data will resume once the technical issues have been resolved.
Additional pneumonia and influenza mortality surveillance information for current and past seasons:
Surveillance Methods | FluView Interactive
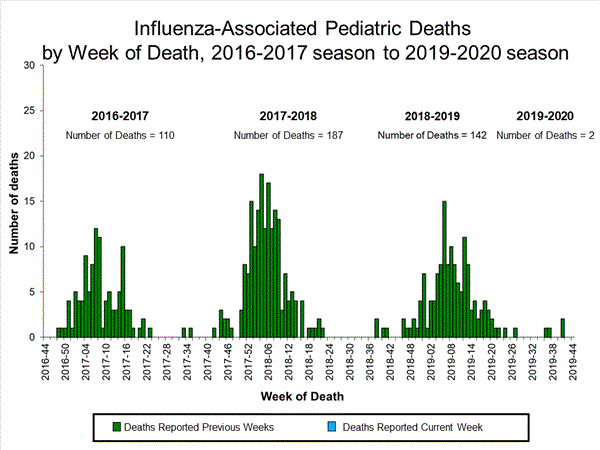
Influenza-Associated Pediatric Mortality
No influenza-associated pediatric deaths were reported to CDC during week 44.
A total of two influenza-associated pediatric deaths occurring during the 2019-2020 season have been reported to CDC.
Additional Influenza-associated pediatric mortality surveillance information for current and past seasons:
Surveillance Methods | FluView Interactive
Additional National and International Influenza Surveillance Information
FluView Interactive: FluView includes enhanced web-based interactive applications that can provide dynamic visuals of the influenza data collected and analyzed by CDC. These FluView Interactive applications allow people to create customized, visual interpretations of influenza data, as well as make comparisons across flu seasons, regions, age groups and a variety of other demographics. To access these tools, visit http://www.cy118119.com/flu/weekly/fluviewinteractive.htm
National Institute for Occupational Safety and Health: Monthly surveillance data on the prevalence of health-related workplace absenteeism among full-time workers in the United States are available from NIOSH at http://www.cy118119.com/niosh/topics/absences/default.html
U.S. State and local influenza surveillance: Click on a jurisdiction below to access the latest local influenza information
World Health Organization: Additional influenza surveillance information from participating WHO member nations is available through FluNet and the Global Epidemiology Reports.
WHO Collaborating Centers for Influenza located in Australia, China, Japan, the United Kingdom, and the United States (CDC in Atlanta, Georgia).
Europe: For the most recent influenza surveillance information from Europe, please see WHO/Europe and the European Centre for Disease Prevention and Control at http://www.flunewseurope.org/.
Public Health Agency of Canada: The most up-to-date influenza information from Canada is available at http://www.phac-aspc.gc.ca/fluwatch/
Public Health England: The most up-to-date influenza information from the United Kingdom is available at https://www.gov.uk/government/statistics/weekly-national-flu-reports
Any links provided to non-Federal organizations are provided solely as a service to our users. These links do not constitute an endorsement of these organizations or their programs by CDC or the Federal Government, and none should be inferred. CDC is not responsible for the content of the individual organization web pages found at these links.
An overview of the CDC influenza surveillance system, including methodology and detailed descriptions of each data component, is available at: http://www.cy118119.com/flu/weekly/overview.htm.
--------------------------------------------------------------------------------