Error processing SSI file

2013-2014 Influenza Season Week 50 ending December 14, 2013
All data are preliminary and may change as more reports are received.
Synopsis:
During week 50 (December 8-14, 2013), influenza activity continued to increase in the United States.
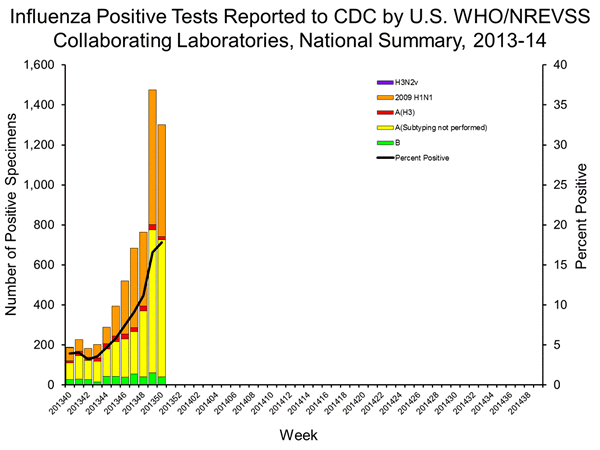
- Viral Surveillance: Of 7,294 specimens tested and reported by U.S. World Health Organization (WHO) and National Respiratory and Enteric Virus Surveillance System (NREVSS) collaborating laboratories during week 50, 1,301 (17.8%) were positive for influenza.
- Pneumonia and Influenza Mortality: The proportion of deaths attributed to pneumonia and influenza (P&I) was below the epidemic threshold.
- Influenza-Associated Pediatric Deaths: Two influenza-associated pediatric deaths were reported, one of which occurred during the 2012-13 season.
- Influenza-associated Hospitalizations: A cumulative rate for the season of 3.0 laboratory-confirmed influenza-associated hospitalizations per 100,000 population was reported.
- Outpatient Illness Surveillance: The proportion of outpatient visits for influenza-like illness (ILI) was 2.3%, above the national baseline of 2.0%. Five regions reported ILI at or above region-specific baseline levels. Four states experienced high ILI activity, one state experienced moderate ILI activity; six states and New York City experienced low ILI activity, 37 states experienced minimal ILI activity and the District of Columbia and two states had insufficient data.
- Geographic Spread of Influenza: The geographic spread of influenza in 4 states was reported as widespread; 20 states reported regional influenza activity; 17 states reported local influenza activity; the District of Columbia, Guam, Puerto Rico, and 8 states reported sporadic influenza activity; one state reported no influenza activity, and the U.S. Virgin Islands did not report.
A description of surveillance methods is available at: http://www.cy118119.com/flu/weekly/overview.htm
| HHS Surveillance Regions* | Data for current week | Data cumulative since September 29, 2013 (Week 40) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Out-patient ILI† | % positive for flu‡ | Number of jurisdictions reporting regional or widespread activity§ | 2009 H1N1 | A (H3) | A(Subtyping not performed) | B | Pediatric Deaths | |||
| Nation | Elevated | 17.8% | 24 of 54 | 2,733 | 229 | 2,847 | 415 | 4 | ||
| Region 1 | Normal | 6.0% | 3 of 6 | 42 | 12 | 12 | 5 | 0 | ||
| Region 2 | Normal | 5.4% | 2 of 4 | 132 | 17 | 42 | 21 | 0 | ||
| Region 3 | Normal | 7.8% | 1 of 6 | 164 | 14 | 2 | 12 | 0 | ||
| Region 4 | Elevated | 19.7% | 8 of 8 | 481 | 6 | 1,875 | 259 | 1 | ||
| Region 5 | Elevated | 20.8% | 2 of 6 | 353 | 22 | 44 | 13 | 1 | ||
| Region 6 | Elevated | 19.4% | 4 of 5 | 521 | 41 | 536 | 43 | 2 | ||
| Region 7 | Elevated | 12.1% | 0 of 4 | 171 | 10 | 17 | 11 | 0 | ||
| Region 8 | Elevated | 17.7% | 2 of 6 | 544 | 22 | 270 | 26 | 0 | ||
| Region 9 | Normal | 9.0% | 1 of 5 | 182 | 56 | 43 | 18 | 0 | ||
| Region 10 | Normal | 12.4% | 1 of 4 | 143 | 29 | 6 | 7 | 0 | ||
*HHS regions (Region 1 CT, ME, MA, NH, RI, VT; Region 2: NJ, NY, Puerto Rico, US Virgin Islands; Region 3: DE, DC, MD, PA, VA, WV; Region 4: AL, FL, GA, KY, MS, NC, SC, TN; Region 5: IL, IN, MI, MN, OH, WI; Region 6: AR, LA, NM, OK, TX; Region 7: IA, KS, MO, NE; Region 8: CO, MT, ND, SD, UT, WY; Region 9: AZ, CA, Guam, HI, NV; and Region 10: AK, ID, OR, WA).
† Elevated means the % of visits for ILI is at or above the national or region-specific baseline
‡ National data are for current week; regional data are for the most recent three weeks
§ Includes all 50 states, the District of Columbia, Guam, Puerto Rico, and U.S. Virgin Islands
U.S. Virologic Surveillance
WHO and NREVSS collaborating laboratories located in all 50 states, Puerto Rico, and Washington D.C. report to CDC the number of respiratory specimens tested for influenza and the number positive by influenza virus type and influenza A virus subtype. The results of tests performed during the current week are summarized in the table below.
Region specific data can be found at http://gis.cdc.gov/grasp/fluview/fluportaldashboard.html.
| Week 50 | |
|---|---|
| No. of specimens tested | 7,294 |
| No. of positive specimens (%) | 1,301 (17.8%) |
| Positive specimens by type/subtype | |
| Influenza A | 1,261 (96.9%) |
| 2009 H1N1 | 559 (44.3%) |
| H3 | 16 (1.3%) |
| Subtyping not performed | 686 (54.4%) |
| Influenza B | 40 (3.1%) |
View National and Regional Level Graphs and Data | View Chart Data | View Full Screen | View PowerPoint Presentation
Antigenic Characterization*
CDC has antigenically characterized 317 influenza viruses [265 2009 H1N1 viruses, 46 influenza A (H3N2) viruses, and 6 influenza B viruses] collected by U.S. laboratories since October 1, 2013 by hemagglutination inhibition (HI).
2009 H1N1 [265]:
- All 265 2009 H1N1 viruses tested were characterized as A/California/7/2009-like, the influenza A (H1N1) component of the 2013-2014 Northern Hemisphere influenza vaccine.
Influenza A (H3N2) [46]:
- All 46 influenza A (H3N2) viruses tested have been characterized as A/Texas/50/2012-like, the influenza A (H3N2) component of the 2013-2014 Northern Hemisphere influenza vaccine.
Influenza B [6]: Two (33%) of the six influenza B viruses tested belong to B/Yamagata/16/88-lineage and the remaining four (67%) influenza B viruses tested belong to B/Victoria/02/87 lineage.
- Yamagata Lineage [2]: Two influenza B/Yamagata-lineage viruses were characterized as B/ Massachusetts/2/1012-like, which is included as an influenza B component of the 2013-2014 Northern Hemisphere trivalent and quadrivalent influenza vaccines.
- Victoria Lineage [4]: Four influenza B/Victoria-lineage viruses were characterized as B/Brisbane/60/2008-like, which is included as an influenza B component of the 2013-2014 Northern Hemisphere quadrivalent influenza vaccine.
Antiviral Resistance
Testing of 2009 H1N1, influenza A (H3N2), and influenza B virus isolates for resistance to neuraminidase inhibitors (oseltamivir and zanamivir) is performed at CDC using a functional assay. Additional 2009 H1N1 and influenza A (H3N2) clinical samples are tested for mutations of the virus known to confer oseltamivir resistance. The data summarized below combine the results of both testing methods. These samples are routinely obtained for surveillance purposes rather than for diagnostic testing of patients suspected to be infected with antiviral-resistant virus.
High levels of resistance to the adamantanes (amantadine and rimantadine) persist among 2009 influenza A (H1N1) and A (H3N2) viruses (the adamantanes are not effective against influenza B viruses). As a result, data from adamantane resistance testing are not presented below.
| Oseltamivir | Zanamivir | |||
|---|---|---|---|---|
| Virus Samples tested (n) | Resistant Viruses, Number (%) | Virus Samples tested (n) | Resistant Viruses, Number (%) | |
| Influenza A (H3N2) | 54 | 0 (0.0) | 54 | 0 (0.0) |
| Influenza B | 9 | 0 (0.0) | 9 | 0 (0.0) |
| 2009 H1N1 | 526* | 7 (1.3) | 323 | 0 (0.0) |
*Includes specimens tested in national surveillance and additional specimens tested at public health laboratories in nine states (AZ, FL, HI, MD, MI, NY, TX, WA, and WI) who share testing results with CDC.
The majority of currently circulating influenza viruses are susceptible to the neuraminidase inhibitor antiviral medications, oseltamivir and zanamivir; however, rare sporadic cases of oseltamivir-resistant 2009 H1N1 and A (H3N2) viruses have been detected worldwide. Antiviral treatment with oseltamivir or zanamivir is recommended as early as possible for patients with confirmed or suspected influenza who have severe, complicated, or progressive illness; who require hospitalization; or who are at greater risk for serious influenza-related complications. Additional information on recommendations for treatment and chemoprophylaxis of influenza virus infection with antiviral agents is available at http://www.cy118119.com/flu/antivirals/index.htm.
Pneumonia and Influenza (P&I) Mortality Surveillance
During week 50, 6.6% of all deaths reported through the 122 Cities Mortality Reporting System were due to P&I. This percentage was below the epidemic threshold of 6.9% for week 50.
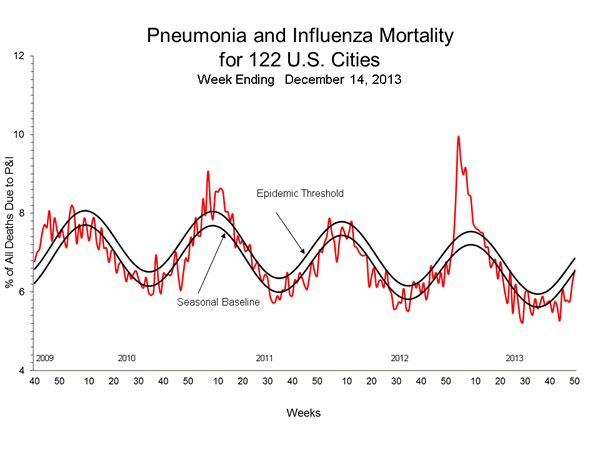
View Full Screen | View PowerPoint Presentation
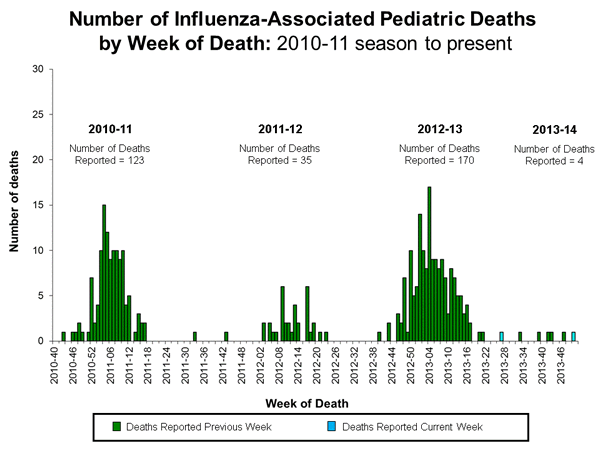
Influenza-Associated Pediatric Mortality
Two influenza-associated pediatric deaths were reported to CDC during week 50. One death was associated with a 2009 H1N1 virus and occurred during the week ending December 14, 2013 (week 50). A total of four influenza-associated pediatric deaths for the 2013-2014 season have been reported.
One death was associated with an influenza A virus for which no subtyping was performed and occurred during the 2012-13 season. This death brings the total number of reported pediatric deaths for that season to 170.
Additional data can be found at http://gis.cdc.gov/GRASP/Fluview/PedFluDeath.html.
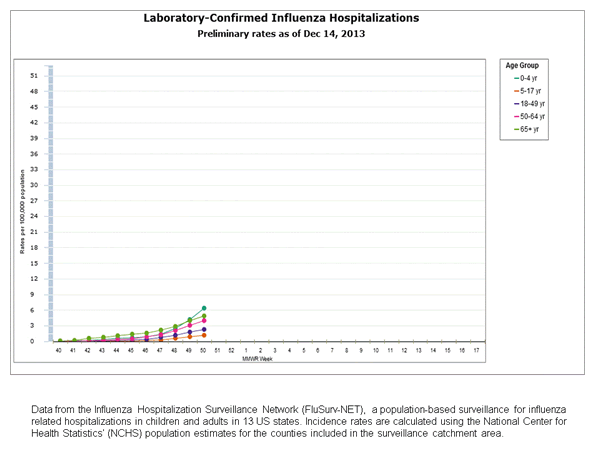
Influenza-Associated Hospitalizations
The The Influenza Hospitalization Surveillance Network (FluSurv-NET) conducts population-based surveillance for laboratory-confirmed influenza-related hospitalizations in children younger than 18 years of age (since the 2003-2004 influenza season) and adults (since the 2005-2006 influenza season).
The FluSurv-NET covers more than 70 counties in the 10 Emerging Infections Program (EIP) states (CA, CO, CT, GA, MD, MN, NM, NY, OR, TN) and additional Influenza Hospitalization Surveillance Project (IHSP) states. The IHSP began during the 2009-2010 season to enhance surveillance during the 2009 H1N1 pandemic. IHSP sites included IA, ID, MI, OK and SD during the 2009-2010 season; ID, MI, OH, OK, RI, and UT during the 2010-2011 season; MI, OH, RI, and UT during the 2011-2012 season; IA, MI, OH, RI, and UT during the 2012-2013 season; and MI, OH, and UT during the 2013-2014 season.
Data gathered are used to estimate age-specific hospitalization rates on a weekly basis, and describe characteristics of persons hospitalized with severe influenza illness. The rates provided are likely to be an underestimate as influenza-related hospitalizations can be missed, either because testing is not performed, or because cases may be attributed to other causes of pneumonia or other common influenza-related complications.
Between October 1, 2013 and December 14, 2013, 815 laboratory-confirmed influenza-associated hospitalizations were reported. This is a rate of 3.0 per 100,000 population. Among cases, 744 (91.3%) were influenza A, 61 (7.5%) were influenza B, 6 (0.7%) were influenza A and B co-infection, and 4 (0.5%) had no virus type information. Among those with influenza A subtype information, 7 (2.3%) were H3 and 297 (97.1%) were 2009 H1N1. The most commonly reported underlying medical conditions among adults were obesity, metabolic disorders, cardiovascular disease, and chronic lung disease (excluding asthma). The most commonly reported underlying medical conditions in children were obesity, asthma, cardiovascular disease and neurologic disorders. Approximately 51.4% of hospitalized children had no identified underlying medical conditions. Among 36 hospitalized women of childbearing age (15-44 years), eight were pregnant.
Additional FluSurv-NET data can be found at:http://gis.cdc.gov/GRASP/Fluview/FluHospRates.html and http://gis.cdc.gov/grasp/fluview/FluHospChars.html.
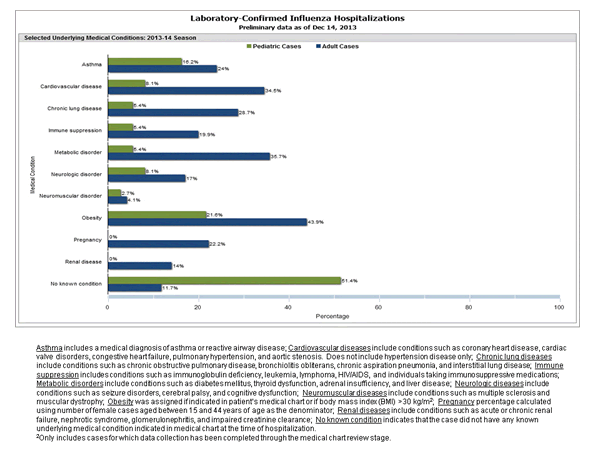
View Interactive Application | View Full Screen | View PowerPoint Presentation
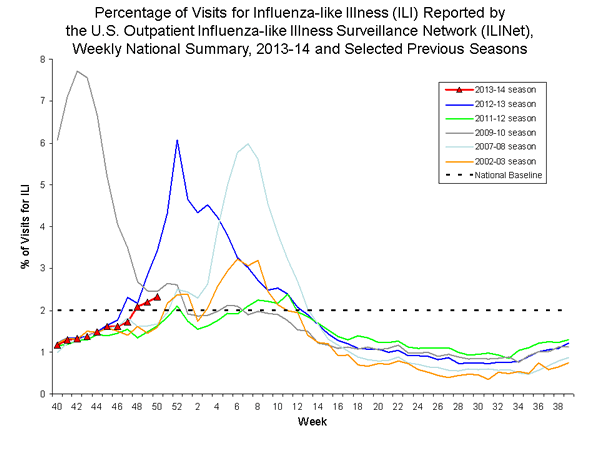
Outpatient Illness Surveillance
Nationwide during week 50, 2.3% of patient visits reported through the U.S. Outpatient Influenza-like Illness Surveillance Network (ILINet) were due to influenza-like illness (ILI). This percentage is above the national baseline of 2.0%. (ILI is defined as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.)
View National and Regional Level Graphs and Data | View
Chart Data | View Full Screen | View PowerPoint Presentation
On a regional level, the percentage of outpatient visits for ILI ranged from 0.8% to 5.8% during week 50. Five regions (Regions 4, 5, 6, 7, and 8) reported a proportion of outpatient visits for ILI at or above their region-specific baseline level.
Region specific data is available at http://gis.cdc.gov/grasp/fluview/fluportaldashboard.html.
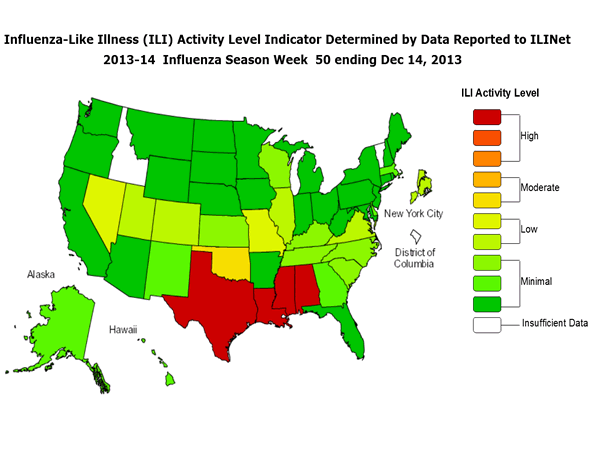
ILINet Activity Indicator Map
Data collected in ILINet are used to produce a measure of ILI activity* by state. Activity levels are based on the percent of outpatient visits in a state due to ILI and are compared to the average percent of ILI visits that occur during weeks with little or no influenza virus circulation. Activity levels range from minimal, which would correspond to ILI activity from outpatient clinics being below, or only slightly above, the average, to high, which would correspond to ILI activity from outpatient clinics being much higher than average.
During week 50, the following ILI activity levels were experienced:
- Four states experienced high ILI activity (Alabama, Louisiana, Mississippi and Texas).
- One state experienced moderate ILI activity (Oklahoma).
- Six states and New York City experienced low ILI activity (Colorado, Illinois, Missouri, Nevada, Utah, and Virginia).
- Thirty-seven states experienced minimal ILI activity (Alaska, Arizona, Arkansas, California, Connecticut, Delaware, Florida, Georgia, Hawaii, Indiana, Iowa, Kansas, Kentucky, Maine, Maryland, Massachusetts, Michigan, Minnesota, Montana, Nebraska, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oregon, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Washington, West Virginia, Wisconsin, and Wyoming).
- Data were insufficient to calculate an ILI activity level from the District of Columbia and two states (Idaho and Vermont).
*This map uses the proportion of outpatient visits to health care providers for influenza-like illness to measure the ILI activity level within a state. It does not, however, measure the extent of geographic spread of flu within a state. Therefore, outbreaks occurring in a single city could cause the state to display high activity levels.
Data collected in ILINet may disproportionately represent certain populations within a state, and therefore, may not accurately depict the full picture of influenza activity for the whole state.
Data displayed in this map are based on data collected in ILINet, whereas the State and Territorial flu activity map is based on reports from state and territorial epidemiologists. The data presented in this map is preliminary and may change as more data is received.
Differences in the data presented here by CDC and independently by some state health departments likely represent differing levels of data completeness with data presented by the state likely being the more complete.
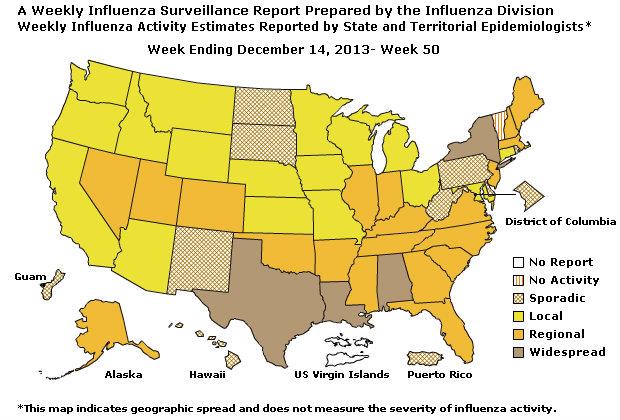
Geographic Spread of Influenza as Assessed by State and Territorial Epidemiologists
The influenza activity reported by state and territorial epidemiologists indicates geographic spread of influenza viruses, but does not measure the severity of influenza activity.
During week 50, the following influenza activity was reported:
- Widespread influenza activity was reported by four states (Alabama, Louisiana, New York, and Texas).
- Regional influenza activity was reported by 20 states (Alaska, Arkansas, Colorado, Florida, Georgia, Illinois, Indiana, Kentucky, Maine, Massachusetts, Mississippi, Nevada, New Hampshire, New Jersey, North Carolina, Oklahoma, South Carolina, Tennessee, Utah, and Virginia).
- Local influenza activity was reported by 17 states (Arizona, California, Connecticut, Idaho, Iowa, Kansas, Maryland, Michigan, Minnesota, Missouri, Montana, Nebraska, Ohio, Oregon, Washington, Wisconsin, and Wyoming).
- Sporadic influenza activity was reported by The District of Columbia, Guam, Puerto Rico, and eight states (Delaware, Hawaii, New Mexico, North Dakota, Pennsylvania, Rhode Island, South Dakota, and West Virginia).
- One state (Vermont) reported no influenza activity.
- The U.S. Virgin Islands did not report.
Flu Activity data in XML Format | View Full Screen
Additional National and International Influenza Surveillance Information
FluView Interactive: FluView includes enhanced web-based interactive applications that can provide dynamic visuals of the influenza data collected and analyzed by CDC. These FluView Interactive applications allow people to create customized, visual interpretations of influenza data, as well as make comparisons across flu seasons, regions, age groups and a variety of other demographics. To access these tools visit http://www.cy118119.com/flu/weekly/fluviewinteractive.htm.
U.S. State and local influenza surveillance: Click on a jurisdiction below to access the latest local influenza information.
Google Flu Trends: Google Flu Trends uses aggregated Google search data in a model created in collaboration with CDC to estimate influenza activity in the United States. For more information and activity estimates from the U.S. and worldwide, see http://www.google.org/flutrends/
World Health Organization: Additional influenza surveillance information from participating WHO member nations is available through FluNet and the Global Epidemiology Reports.
WHO Collaborating Centers for Influenza located in Australia, China, Japan, and the United Kingdom.
Europe: for the most recent influenza surveillance information from Europe, please see WHO/Europe at http://www.euroflu.org/index.php and visit the European Centre for Disease Prevention and Control at http://ecdc.europa.eu/en/publications/surveillance_reports/influenza/Pages/weekly_influenza_surveillance_overview.aspx
Public Health Agency of Canada: The most up-to-date influenza information from Canada is available at http://www.phac-aspc.gc.ca/fluwatch/
Health Protection Agency (United Kingdom): The most up-to-date influenza information from the United Kingdom is available at http://www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/SeasonalInfluenza/
Any links provided to non-Federal organizations are provided solely as a service to our users. These links do not constitute an endorsement of these organizations or their programs by CDC or the Federal Government, and none should be inferred. CDC is not responsible for the content of the individual organization web pages found at these links.
--------------------------------------------------------------------------------
A description of surveillance methods is available at: http://www.cy118119.com/flu/weekly/overview.htm