Error processing SSI file

2012-2013 Influenza Season Week 50 ending December 15, 2012
All data are preliminary and may change as more reports are received.
Synopsis:
During week 50 (December 9-15), influenza activity increased in the U.S.
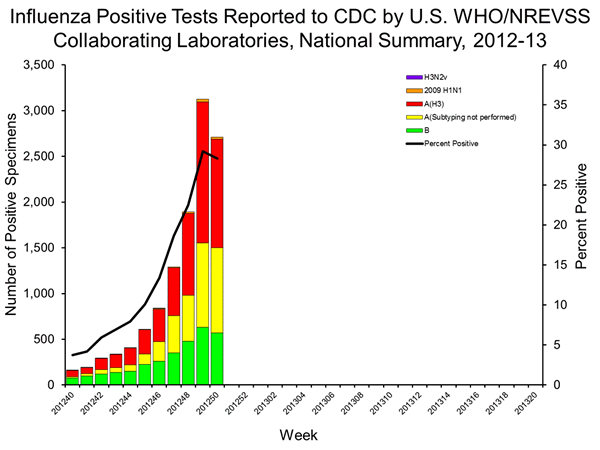
- Viral Surveillance: Of 9,562 specimens tested and reported by U.S. World Health Organization (WHO) and National Respiratory and Enteric Virus Surveillance System (NREVSS) collaborating laboratories in week 50, 2,709 (28.3%) were positive for influenza.
- Pneumonia and Influenza Mortality: The proportion of deaths attributed to pneumonia and influenza (P&I) was below the epidemic threshold.
- Influenza-Associated Pediatric Deaths: Two influenza-associated pediatric deaths were reported. One was associated with an influenza A (H3) virus and one was associated with an influenza A virus for which the subtype was not determined.
- Outpatient Illness Surveillance: The proportion of outpatient visits for influenza-like illness (ILI) was 3.2%; above the national baseline of 2.2%. Nine of ten regions reported ILI above region-specific baseline levels. Twelve states experienced high ILI activity, New York City and 5 states experienced moderate ILI activity; 11 states experienced low ILI activity; 22 states experienced minimal ILI activity, and the District of Columbia had insufficient data.
- Geographic Spread of Influenza: Twenty-nine states reported widespread geographic influenza activity; 12 states reported regional activity; the District of Columbia and 5 states reported local activity; 3 states reported sporadic activity; Guam reported no influenza activity, and Puerto Rico, the U.S. Virgin Islands, and 1 state did not report.
A description of surveillance methods is available at: http://www.cy118119.com/flu/weekly/overview.htm
| HHS Surveillance Regions* | Data for current week | Data cumulative since September 30, 2012 (Week 40) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Out-patient ILI† | % positive for flu‡ | Number of jurisdictions reporting regional or widespread activity§ | 2009 H1N1 | A (H3) | A(Subtyping not performed) | B | Pediatric Deaths | |||
| Nation | Elevated | 28.3% | 41 of 54 | 110 | 5,351 | 3,293 | 3,103 | 8 | ||
| Region 1 | Elevated | 27.1% | 5 of 6 | 2 | 220 | 55 | 22 | 0 | ||
| Region 2 | Elevated | 21.8% | 2 of 4 | 17 | 306 | 251 | 128 | 2 | ||
| Region 3 | Elevated | 32.2% | 4 of 6 | 24 | 825 | 63 | 86 | 0 | ||
| Region 4 | Elevated | 29.5% | 8 of 8 | 15 | 941 | 2,133 | 801 | 3 | ||
| Region 5 | Elevated | 50.8% | 6 of 6 | 21 | 923 | 124 | 260 | 1 | ||
| Region 6 | Elevated | 19.4% | 4 of 5 | 5 | 314 | 448 | 668 | 2 | ||
| Region 7 | Elevated | 29.5% | 4 of 4 | 1 | 561 | 75 | 309 | 0 | ||
| Region 8 | Elevated | 23.3% | 5 of 6 | 15 | 369 | 93 | 686 | 0 | ||
| Region 9 | Normal | 10.3% | 0 of 5 | 9 | 258 | 41 | 72 | 0 | ||
| Region 10 | Elevated | 26.0% | 3 of 4 | 1 | 634 | 10 | 71 | 0 | ||
*HHS regions (Region 1 CT, ME, MA, NH, RI, VT; Region 2: NJ, NY, Puerto Rico, US Virgin Islands; Region 3: DE, DC, MD, PA, VA, WV; Region 4: AL, FL, GA, KY, MS, NC, SC, TN; Region 5: IL, IN, MI, MN, OH, WI; Region 6: AR, LA, NM, OK, TX; Region 7: IA, KS, MO, NE; Region 8: CO, MT, ND, SD, UT, WY; Region 9: AZ, CA, Guam, HI, NV; and Region 10: AK, ID, OR, WA).
† Elevated means the % of visits for ILI is at or above the national or region-specific baseline
‡ National data are for current week; regional data are for the most recent three weeks
§ Includes all 50 states, the District of Columbia, Guam, Puerto Rico, and U.S. Virgin Islands
U.S. Virologic Surveillance:
WHO and NREVSS collaborating laboratories located in all 50 states and Puerto Rico report to CDC the number of respiratory specimens tested for influenza and the number positive by influenza virus type and influenza A virus subtype. Region specific data can be found at http://gis.cdc.gov/grasp/fluview/fluportaldashboard.html. The results of tests performed during the current week are summarized in the table below.
| Week 50 | |
|---|---|
| No. of specimens tested | 9,562 |
| No. of positive specimens (%) | 2,709 (28.3%) |
| Positive specimens by type/subtype | |
| Influenza A | 2,138 (78.9%) |
| 2009 H1N1 | 22 (1.0%) |
| Subtyping not performed | 929 (43.5%) |
| H3 | 1,187 (55.5%) |
| Influenza B | 571 (21.1%) |
View National and Regional Level Graphs and Data | View Chart Data | View Full Screen | View PowerPoint Presentation
Since the start of the season, influenza A (H3N2) viruses have predominated nationally, followed by influenza B viruses; 2009 H1N1 viruses have been identified rarely. The predominant circulating virus has varied by state and by region.
Antigenic Characterization:
CDC has antigenically characterized 351 influenza viruses [Ten 2009 H1N1 viruses, 226 influenza A (H3N2) viruses, and 115 influenza B viruses] collected by U.S. laboratories since October 1, 2012.
2009 H1N1 [10]:
- All ten 2009 H1N1 viruses tested were characterized as A/California/7/2009-like, the influenza A (H1N1) component of the 2012-2013 influenza vaccine for the Northern Hemisphere.
Influenza A (H3N2) [226]:
- 224 (99.1%) of the 226 H3N2 influenza viruses tested have been characterized as A/Victoria/361/2011-like, the influenza A (H3N2) component of the 2012-2013 Northern Hemisphere influenza vaccine.
- Two (0.9%) of the 226 H3N2 viruses tested showed reduced titers with antiserum produced against A/Victoria/361/2011.
Influenza B (B/Yamagata/16/88 and B/Victoria/02/87 lineages) [115]:
- Yamagata Lineage [79]: 79 (68.7%) of the 115 influenza B viruses tested so far this season have been characterized as B/Wisconsin/1/2010-like, the influenza B component of the 2012-2013 Northern Hemisphere influenza vaccine.
- Victoria Lineage [36]: 36 (31.3%) of 115 influenza B viruses tested have been from the B/Victoria lineage of viruses.
Antiviral Resistance:
Testing of 2009 H1N1, influenza A (H3N2), and influenza B virus isolates for resistance to neuraminidase inhibitors (oseltamivir and zanamivir) is performed at CDC using a functional assay. Additional 2009 influenza A (H1N1) clinical samples are tested for a single mutation in the neuraminidase of the virus known to confer oseltamivir resistance (H275Y). The data summarized below combine the results of both testing methods. These samples are routinely obtained for surveillance purposes rather than for diagnostic testing of patients suspected to be infected with antiviral-resistant virus.
High levels of resistance to the adamantanes (amantadine and rimantadine) persist among 2009 influenza A (H1N1) and A (H3N2) viruses (the adamantanes are not effective against influenza B viruses). As a result, data from adamantane resistance testing are not presented below.
| Oseltamivir | Zanamivir | |||
|---|---|---|---|---|
| Virus Samples tested (n) | Resistant Viruses, Number (%) | Virus Samples tested (n) | Resistant Viruses, Number (%) | |
| Influenza A (H3N2) | 344 | 0 (0.0) | 344 | 0 (0.0) |
| Influenza B | 135 | 0 (0.0) | 135 | 0 (0.0) |
| 2009 H1N1 | 18 | 0 (0.0) | 15 | 0 (0.0) |
The majority of currently circulating influenza viruses are susceptible to the neuraminidase inhibitor antiviral medications oseltamivir and zanamivir; however, rare sporadic cases of oseltamivir-resistant 2009 H1N1 and A (H3N2) viruses have been detected worldwide. Antiviral treatment with oseltamivir or zanamivir is recommended as early as possible for patients with confirmed or suspected influenza who have severe, complicated, or progressive illness; who require hospitalization; or who are at greater risk for serious influenza-related complications. Additional information on recommendations for treatment and chemoprophylaxis of influenza virus infection with antiviral agents is available at http://www.cy118119.com/flu/antivirals/index.htm.
Novel Influenza A Virus:
No new human infections with novel influenza A viruses were reported to CDC during week 50.
A total of 312 infections with variant influenza viruses (308 H3N2v viruses, 3 H1N2v viruses, and 1 H1N1v virus) have been reported from 11 states since July 2012. More information about H3N2v infections can be found at http://www.cy118119.com/flu/swineflu/h3n2v-outbreak.htm.
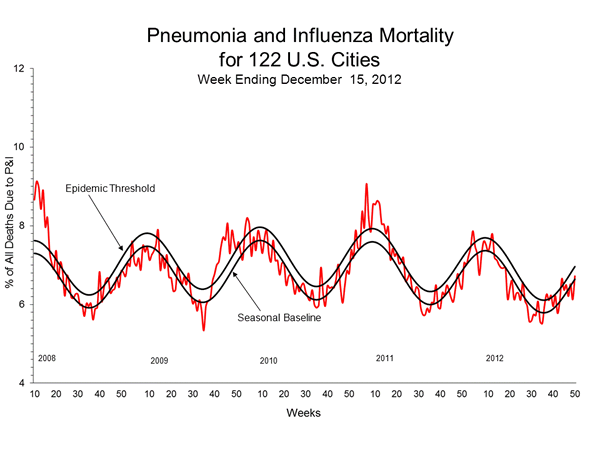
Pneumonia and Influenza (P&I) Mortality Surveillance:
During week 50, 6.7% of all deaths reported through the 122 Cities Mortality Reporting System were due to P&I. This percentage was below the epidemic threshold of 7.0% for week 50.
View Full Screen | View PowerPoint Presentation
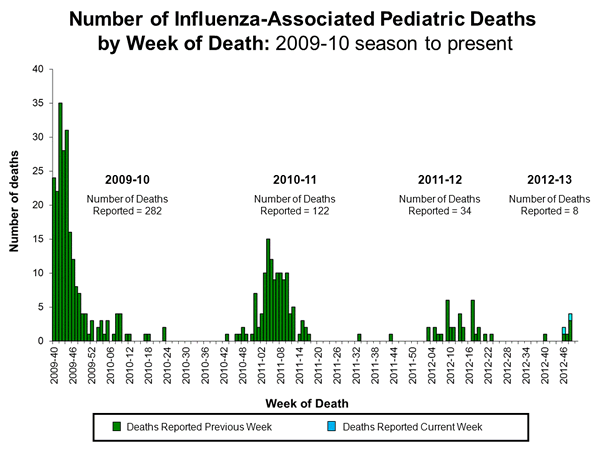
Influenza-Associated Pediatric Mortality:
Two influenza-associated pediatric deaths were reported to CDC during week 50. One was associated with an influenza A (H3) virus and one was associated with an influenza A virus for which the subtype was not determined. These deaths occurred during the weeks ending November 17 (week 46) and December 1 (week 48). This brings the total number of influenza-associated pediatric deaths reported during the 2012-2013 season to 8. Additional data can be found at http://gis.cdc.gov/GRASP/Fluview/PedFluDeath.html.
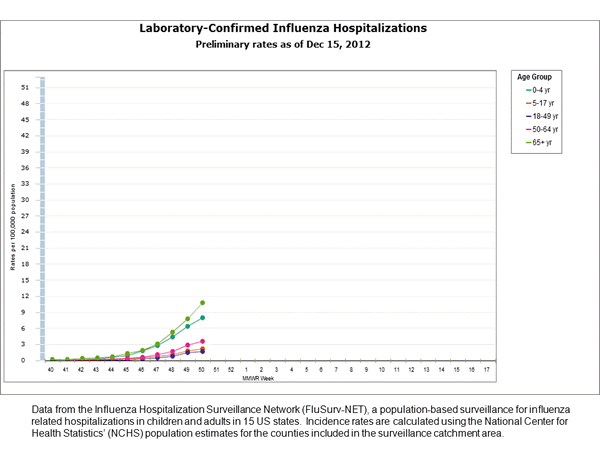
Influenza-Associated Hospitalizations:
The Influenza Hospitalization Surveillance Network (FluSurv-NET) conducts population-based surveillance for laboratory-confirmed influenza-related hospitalizations in children younger than 18 years of age (since the 2003-2004 influenza season) and adults (since the 2005-2006 influenza season).
The FluSurv-NET covers more than 80 counties in the 10 Emerging Infections Program (EIP) states (CA, CO, CT, GA, MD, MN, NM, NY, OR, TN) and additional Influenza Hospitalization Surveillance Project (IHSP) states. The IHSP began during the 2009-2010 season to enhance surveillance during the 2009 H1N1 pandemic. IHSP sites included IA, ID, MI, OK and SD during the 2009-2010 season; ID, MI, OH, OK, RI, and UT during the 2010-2011 season; MI, OH, RI, and UT during the 2011-2012 season; and IA, MI, OH, RI, and UT during the 2012-2013 season.
Data gathered are used to estimate age-specific hospitalization rates on a weekly basis, and describe characteristics of persons hospitalized with severe influenza illness. The rates provided are likely to be an underestimate as influenza-related hospitalizations can be missed, either because testing is not performed, or because cases may be attributed to other causes of pneumonia or other common influenza-related complications.
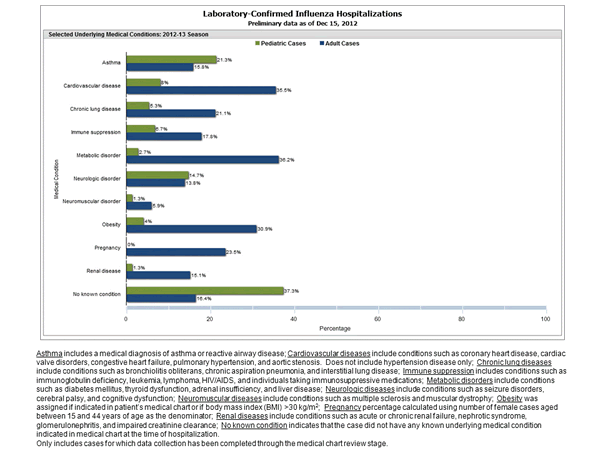
Between October 1, 2012 and December 15, 2012, 1,013 laboratory-confirmed influenza-associated hospitalizations were reported. This is a rate of 3.6 per 100,000 population. Among all hospitalizations, 811 (80.1%) were associated with influenza A and 188 (18.6%) with influenza B. There was no virus type information for 13 (1.3%) hospitalizations. Among hospitalizations with influenza A subtype information, 218 (97.3%) were attributed to H3 and 6 (2.7%) were attributed to 2009 H1N1. The most commonly reported underlying medical conditions among hospitalized adults were metabolic conditions, cardiovascular disease, obesity, and chronic lung disease (excluding asthma). Among 17 hospitalized women of childbearing age (15-44 years), four were pregnant. The most commonly reported underlying medical conditions in hospitalized children were asthma, neurologic disorders, cardiovascular disease, and immune suppression. Approximately 40% of hospitalized children had no identified underlying medical conditions. Additional FluSurv-NET data can be found at: http://gis.cdc.gov/GRASP/Fluview/FluHospRates.html and http://gis.cdc.gov/grasp/fluview/FluHospChars.html.
View Interactive Application | View Full Screen | View PowerPoint Presentation
Outpatient Illness Surveillance:
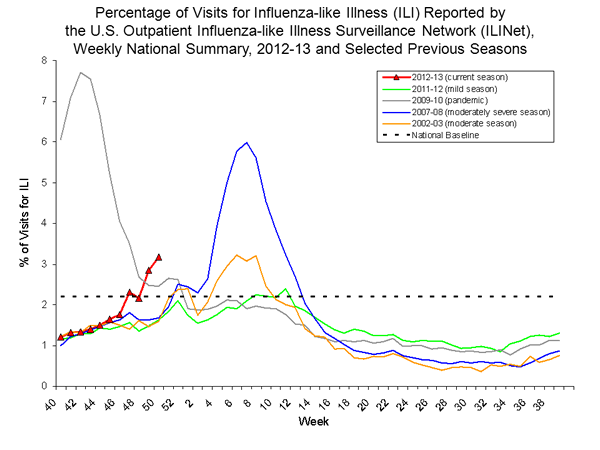
Nationwide during week 50, 3.2% of patient visits reported through the U.S. Outpatient Influenza-like Illness Surveillance Network (ILINet) were due to influenza-like illness (ILI). This percentage is above the national baseline of 2.2%. (ILI is defined as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.) Region specific data is available at http://gis.cdc.gov/grasp/fluview/fluportaldashboard.html.
View National and Regional Level Graphs and Data | View Chart Data | View Full Screen | View PowerPoint Presentation
On a regional level, the percentage of outpatient visits for ILI ranged from 1.1% to 4.8% during week 50. Nine regions (Regions 1 - 8 and 10) reported a proportion of outpatient visits for ILI above their region-specific baseline levels.
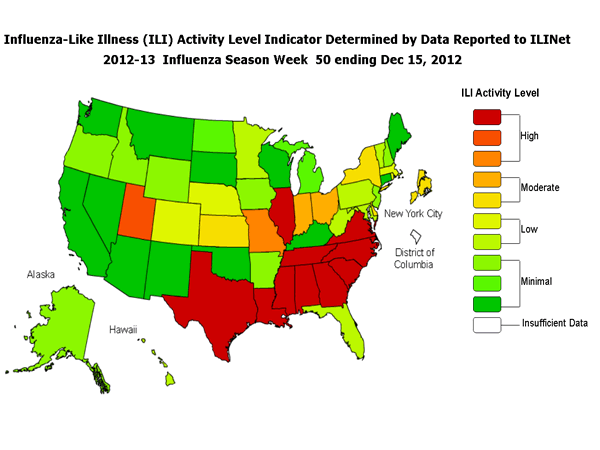
ILINet Activity Indicator Map:
Data collected in ILINet are used to produce a measure of ILI activity* by state. Activity levels are based on the percent of outpatient visits in a state due to ILI and are compared to the average percent of ILI visits that occur during spring and fall weeks with little or no influenza virus circulation. Activity levels range from minimal, which would correspond to ILI activity from outpatient clinics being below the average, to high, which would correspond to ILI activity from outpatient clinics being much higher than average.
During week 50, the following ILI activity levels were experienced:
- Twelve states experienced high ILI activity (Alabama, Georgia, Illinois, Louisiana, Mississippi, Missouri, North Carolina, South Carolina, Tennessee, Texas, Utah, and Virginia).
- New York City and 5 states experienced moderate ILI activity (Delaware, Indiana, Kansas, New York, and Ohio).
- Eleven states experienced low ILI activity (Colorado, Florida, Hawaii, Maryland, Massachusetts, Minnesota, Nebraska, Pennsylvania, Rhode Island, Vermont, and West Virginia).
- Twenty-two states experienced minimal ILI activity (Alaska, Arizona, Arkansas, California, Connecticut, Idaho, Iowa, Kentucky, Maine, Michigan, Montana, Nevada, New Hampshire, New Jersey, New Mexico, North Dakota, Oklahoma, Oregon, South Dakota, Washington, Wisconsin, and Wyoming).
- Data were insufficient to calculate an ILI activity level for the District of Columbia.
*This map uses the proportion of outpatient visits to health care providers for influenza-like illness to measure the ILI activity level within a state. It does not, however, measure the extent of geographic spread of flu within a state. Therefore, outbreaks occurring in a single city could cause the state to display high activity levels.
Data collected in ILINet may disproportionately represent certain populations within a state, and therefore, may not accurately depict the full picture of influenza activity for the whole state.
Data displayed in this map are based on data collected in ILINet, whereas the State and Territorial flu activity map are based on reports from state and territorial epidemiologists. The data presented in this map is preliminary and may change as more data is received.
Differences in the data presented here by CDC and independently by some state health departments likely represent differing levels of data completeness with data presented by the state likely being the more complete.
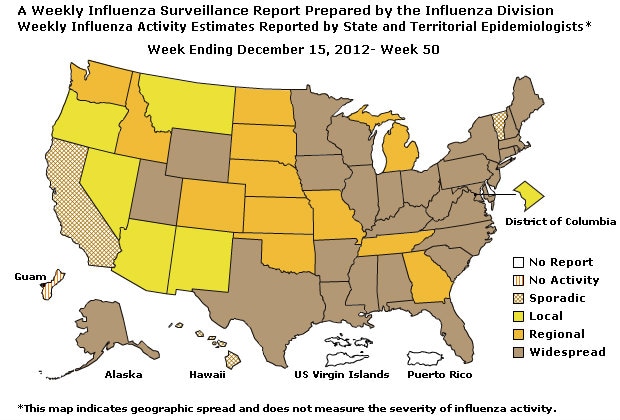
Geographic Spread of Influenza as Assessed by State and Territorial Epidemiologists:
The influenza activity reported by state and territorial epidemiologists indicates geographic spread of influenza viruses, but does not measure the severity of influenza activity.
During week 50, the following influenza activity was reported:
- Widespread influenza activity was reported by 29 states (Alabama, Alaska, Arkansas, Connecticut, Florida, Kentucky, Illinois, Indiana, Iowa, Louisiana, Maine, Maryland, Massachusetts, Minnesota, Mississippi, New Hampshire, New Jersey, New York, North Carolina, Ohio, Pennsylvania, Rhode Island, South Carolina, Texas, Utah, Virginia, West Virginia, Wisconsin, and Wyoming).
- Regional influenza activity was reported by 12 states (Colorado, Georgia, Idaho, Kansas, Michigan, Missouri, Nebraska, North Dakota, Oklahoma, South Dakota, Tennessee, and Washington).
- Local influenza activity was reported by the District of Columbia and 5 states (Arizona, Montana, Nevada, New Mexico, and Oregon).
- Sporadic influenza activity was reported by and 3 states (California, Hawaii, and Vermont).
- Guam reported no influenza activity
- Puerto Rico, the U.S. Virgin Islands, and 1 state (Delaware) did not report.
- Content Source: Coordinating Center for Infectious Diseases (CCID)
- National Center for Immunization and Respiratory Diseases (NCIRD)
Flu Activity data in XML Format | View Full Screen
Additional National and International Influenza Surveillance Information
FluView Interactive: This season, FluView includes enhanced web-based interactive applications that can provide dynamic visuals of the influenza data collected and analyzed by CDC. These FluView Interactive applications allow people to create customized, visual interpretations of influenza data, as well as comparisons across flu seasons, regions, age groups and a variety of other demographics. To access these tools visit http://www.cy118119.com/flu/weekly/fluviewinteractive.htm.
U.S. State and local influenza surveillance: Click on a jurisdiction below to access the latest local influenza information.
Google Flu Trends: Google Flu Trends uses aggregated Google search data in a model created in collaboration with CDC to estimate influenza activity in the United States. For more information and activity estimates from the U.S. and worldwide, see http://www.google.org/flutrends/
World Health Organization: Additional influenza surveillance information from participating WHO member nations is available through FluNet and the Global Epidemiology Reports.
WHO Collaborating Centers for Influenza located in Australia, China, Japan, and the United Kingdom.
Europe: for the most recent influenza surveillance information from Europe, please see WHO/Europe at http://www.euroflu.org/index.php and visit the European Centre for Disease Prevention and Control at http://ecdc.europa.eu/en/publications/surveillance_reports/influenza/Pages/weekly_influenza_surveillance_overview.aspx
Public Health Agency of Canada: The most up-to-date influenza information from Canada is available at http://www.phac-aspc.gc.ca/fluwatch/
Health Protection Agency (United Kingdom): The most up-to-date influenza information from the United Kingdom is available at http://www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/SeasonalInfluenza/
Any links provided to non-Federal organizations are provided solely as a service to our users. These links do not constitute an endorsement of these organizations or their programs by CDC or the Federal Government, and none should be inferred. CDC is not responsible for the content of the individual organization web pages found at these links.
--------------------------------------------------------------------------------
A description of surveillance methods is available at: http://www.cy118119.com/flu/weekly/overview.htm