
2009-2010 Influenza Season Week 46 ending November 21, 2009
All data are preliminary and may change as more reports are received.
Synopsis:
During week 46 (November 15-21, 2009), influenza activity continued to decrease in the U.S.
- 1,880 (20.5%) specimens tested by U.S. World Health Organization (WHO) and National Respiratory and Enteric Virus Surveillance System (NREVSS) collaborating laboratories and reported to CDC/Influenza Division were positive for influenza.
- Over 99% of all subtyped influenza A viruses being reported to CDC were 2009 influenza A (H1N1) viruses.
- The proportion of deaths attributed to pneumonia and influenza (P&I) was above the epidemic threshold for the eighth consecutive week.
- Thirty-five influenza-associated pediatric deaths were reported. Twenty-seven of these deaths were associated with 2009 influenza A (H1N1) virus infection, seven were associated with an influenza A virus for which the subtype was undetermined, and one was associated with a seasonal influenza A (H1) virus infection that occurred in March.
- The proportion of outpatient visits for influenza-like illness (ILI) was 4.3% which is above the national baseline of 2.3%. All 10 regions reported ILI above region-specific baseline levels.
- Thirty-two states reported geographically widespread influenza activity, Puerto Rico and 17 states reported regional influenza activity, the District of Columbia and one state reported local influenza activity, and Guam and the U.S. Virgin Islands reported sporadic influenza activity.
HHS Surveillance Regions* |
Data for current week | Data cumulative for the season | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Out-patient ILI? | % positive for flu? | Number of jurisdictions reporting regional or widespread activity§ | A (H1) | A (H3) | 2009 A (H1N1) | A (unable to sub-type)¥ | A(Subtyping not performed) | B | Pediatric Deaths | |
| Nation | Elevated | 20.5 % | 50 of 54 | 24 | 41 | 53,291 | 400 | 17,552 | 162 | 172 |
| Region 1 | Elevated | 37.0 % | 6 of 6 | 5 | 2 | 2,557 | 8 | 404 | 9 | 4 |
| Region 2 | Elevated | 27.3 % | 3 of 4 | 1 | 5 | 852 | 0 | 859 | 3 | 5 |
| Region 3 | Elevated | 47.0 % | 5 of 6 | 3 | 6 | 9,446 | 34 | 1,354 | 14 | 11 |
| Region 4 | Elevated | 19.5 % | 8 of 8 | 0 | 3 | 5,808 | 89 | 3,840 | 37 | 37 |
| Region 5 | Elevated | 35.4 % | 6 of 6 | 6 | 16 | 8,073 | 45 | 1,222 | 11 | 19 |
| Region 6 | Elevated | 11.5 % | 5 of 5 | 0 | 3 | 2,580 | 19 | 4,251 | 27 | 58 |
| Region 7 | Elevated | 19.7 % | 4 of 4 | 4 | 1 | 3,162 | 148 | 903 | 3 | 3 |
| Region 8 | Elevated | 23.2 % | 5 of 6 | 3 | 1 | 9,173 | 0 | 3,569 | 50 | 11 |
| Region 9 | Elevated | 25.7 % | 4 of 5 | 0 | 3 | 7,273 | 44 | 968 | 6 | 15 |
| Region 10 | Elevated | 40.6 % | 4 of 4 | 2 | 1 | 4,367 | 13 | 182 | 2 | 9 |
*Influenza season officially begins each year at week 40. This season data from week 35 will be included to show the trend of influenza activity before the official start of the 2009-10 influenza season.
**HHS regions (Region 1 CT, ME, MA, NH, RI, VT; Region 2: NJ, NY, Puerto Rico, US Virgin Islands; Region 3: DE, DC, MD, PA, VA, WV; Region 4: AL, FL, GA, KY, MS, NC, SC, TN; Region 5: IL, IN, MI, MN, OH, WI; Region 6: AR, LA, NM, OK, TX; Region 7: IA, KS, MO, NE; Region 8: CO, MT, ND, SD, UT, WY; Region 9: AZ, CA, Guam, HI, NV; and Region 10: AK, ID, OR, WA).
† Elevated means the % of visits for ILI is at or above the national or region-specific baseline
‡ National data are for current week; regional data are for the most recent three weeks
§ Includes all 50 states, the District of Columbia, Guam, Puerto Rico, and U.S. Virgin Islands
¥ The majority of influenza A viruses that cannot be sub-typed as seasonal influenza viruses are 2009 A (H1N1) influenza viruses upon further testing
U.S. Virologic Surveillance:
WHO and NREVSS collaborating laboratories located in all 50 states and Washington D.C., report to CDC the number of respiratory specimens tested for influenza and the number positive by influenza type and subtype. The results of tests performed during the current week are summarized in the table below.
| Week 46 | |
|---|---|
| No. of specimens tested | 9,159 |
| No. of positive specimens (%) | 1,880 (20.5%) |
| Positive specimens by type/subtype | |
| Influenza A | 1,874 (99.7%) |
| A (2009 H1N1) | 1,478 (78.9%) |
| A (subtyping not performed) | 372 (19.9%) |
| A (unable to subtype) | 23 (1.2%) |
| A (H3) | 0 (0.0%) |
| A (H1) | 1 (0.1%) |
| Influenza B | 6 (0.3%) |
During week 46, seasonal influenza A (H1N1) and influenza B viruses co-circulated at low levels with 2009 influenza A (H1N1) viruses. Over 99% of all subtyped influenza A viruses reported to CDC this week were 2009 influenza A (H1N1) viruses.
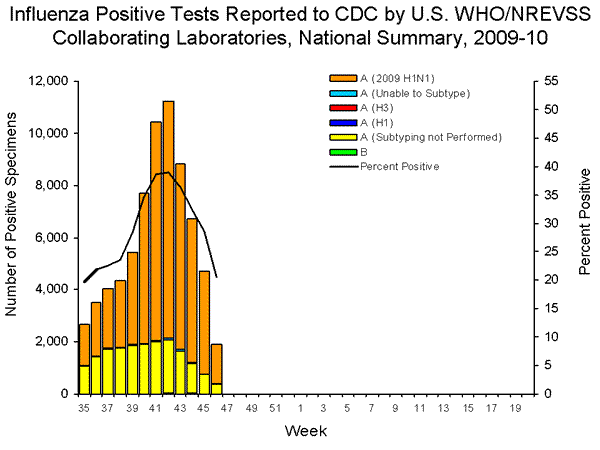
View WHO-NREVSS Regional Bar Charts | View Chart Data | View Full Screen | View PowerPoint Presentation
Pneumonia and Influenza Hospitalization and Death Tracking:
This new system was implemented on August 30, 2009, and replaces the weekly report of laboratory confirmed 2009 H1N1-related hospitalizations and deaths that began in April 2009. Jurisdictions can now report to CDC counts of hospitalizations and deaths resulting from all types or subtypes of influenza, not just those from 2009 H1N1 influenza virus. To allow jurisdictions to implement the new case definition, counts were reset to zero on August 30, 2009. From August 30 – November 21, 2009, 29,348 laboratory-confirmed influenza-associated hospitalizations and 1,224 laboratory-confirmed influenza-associated deaths were reported to CDC. CDC will continue to use its traditional surveillance systems to track the progress of the 2009-10 influenza season.
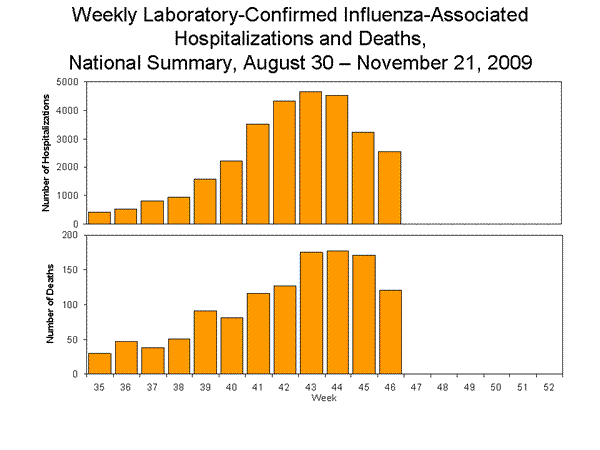
View Full Screen | View PowerPoint Presentation
Antigenic Characterization:
CDC has antigenically characterized one seasonal influenza A (H1N1), three influenza A (H3N2), four influenza B, and 412 2009 influenza A (H1N1) viruses collected since September 1, 2009.
One seasonal influenza A (H1N1) virus was tested and is related to the influenza A (H1N1) component of the 2009-10 Northern Hemisphere influenza vaccine (A/Brisbane/59/2007).
The three influenza A (H3N2) viruses tested showed reduced titers with antisera produced against A/Brisbane/10/2007, the 2009-2010 Northern Hemisphere influenza A (H3N2) vaccine component, and were antigenically related to A/Perth/16/2009, the WHO recommended influenza A (H3N2) component of the 2010 Southern Hemisphere vaccine formulation.
Influenza B viruses currently circulating globally can be divided into two distinct lineages represented by the B/Yamagata/16/88 and B/Victoria/02/87 viruses. The influenza B component of the 2009-10 vaccine belongs to the B/Victoria lineage. The four influenza B viruses tested belong to the B/Victoria lineage and are related to the influenza vaccine component for the 2009-10 Northern Hemisphere influenza vaccine (B/Brisbane/60/2008).
Four hundred eleven (99.8%) of 412 2009 influenza A (H1N1) viruses tested are related to the A/California/07/2009 (H1N1) reference virus selected by WHO as the 2009 H1N1 vaccine virus and one virus (0.2%) tested showed a reduced titer with antiserum produced against A/California/07/2009.
Annual influenza vaccination is expected to provide the best protection against those virus strains that are related to the vaccine strains, but limited to no protection may be expected when the vaccine and circulating virus strains are so different as to be from different lineages. Antigenic characterization of 2009 influenza A(H1N1) viruses indicates that these viruses are only distantly related antigenically and genetically to seasonal influenza A(H1N1) viruses, suggesting that little to no protection would be expected from vaccination with seasonal influenza vaccine. It is too early in the influenza season to determine if seasonal influenza viruses will circulate widely or how well the seasonal vaccine and circulating strains will match.
Antiviral Resistance:
Since September 1, 2009, five influenza A (H3N2), one influenza B, and 402 2009 influenza A (H1N1) virus isolates have been tested for resistance to the neuraminidase inhibitors (oseltamivir and zanamivir), and 1,007 2009 influenza A (H1N1) original clinical samples were tested for a single known mutation in the virus that confers oseltamivir resistance. In addition, two influenza A (H3N2) and 207 2009 influenza A (H1N1) virus isolates have been tested for resistance to the adamantanes (amantadine and rimantadine). Additional laboratories perform antiviral testing and report their results to CDC. The results of antiviral resistance testing performed on these viruses are summarized in the table below.
| Samples tested (n) | Resistant Viruses, Number (%) |
Samples tested (n) | Resistant Viruses, Number (%) | Samples tested (n) | Resistant Viruses, Number (%) | |
|---|---|---|---|---|---|---|
| Oseltamivir | Zanamivir | Adamantanes | ||||
| Seasonal Influenza A (H1N1) | 0 | 0 (0) | 0 | 0 (0) | 0 | 0 (0) |
| Influenza A (H3N2) | 5 | 0 (0) | 0 | 0 (0) | 2 | 2 (100) |
| Influenza B | 1 | 0 (0) | 0 | 0 (0) | N/A* | N/A* |
| 2009 Influenza A (H1N1) | 1,409 | 12?? (0.9) | 402 | 0 (0) | 207 | 206 (99.5) |
*The adamantanes (amantadine and rimantadine) are not effective against influenza B viruses.
†Two screening tools were used to determine oseltamivir resistance: sequence analysis of viral genes or a neuraminidase inhibition assay.
‡Additional laboratories perform antiviral resistance testing and report their results to CDC. One additional oseltamivir resistant 2009 influenza A (H1N1) virus has been identified by these laboratories since September 1, 2009, bringing the total number to 13.
Over 99% of all of the subtyped influenza A viruses reported during week 46 were 2009 influenza A (H1N1) viruses, and the majority of 2009 H1N1 viruses tested since April 2009 have been resistant to the adamantanes (amantadine and rimantadine).
Antiviral treatment with oseltamivir or zanamivir is recommended for all patients with confirmed or suspected influenza virus infection who are hospitalized or who are at higher risk for influenza complications. Additional information on antiviral recommendations for treatment and chemoprophylaxis of influenza virus infection is available at http://www.cy118119.com/h1n1flu/recommendations.htm.
2009 influenza A (H1N1) viruses were tested for oseltamivir resistance by a neuraminidase inhibition assay and/or detection of genetic sequence mutation, depending on the type of specimen tested. Original clinical samples were examined for a single known mutation in the virus that confers oseltamivir resistance in currently circulating seasonal influenza A (H1N1) viruses, while influenza virus isolates were tested using a neuraminidase inhibition assay that determines the presence or absence of neuraminidase inhibitor resistance, followed by the neuraminidase gene sequence analysis of resistant viruses.
The majority of 2009 influenza A (H1N1) viruses are susceptible to the neuraminidase inhibitor antiviral medication oseltamivir; however, rare sporadic cases of oseltamivir resistant 2009 influenza A (H1N1) viruses have been detected worldwide. A total of 23 cases of oseltamivir resistant 2009 influenza A (H1N1) viruses have been identified in the United States since April 2009. In specimens collected since September 1, 2009, 13 cases have been identified in the United States, including two newly identified cases since last week. The proportion of oseltamivir-resistant 2009 H1N1 viruses does not represent the prevalence of oseltamivir-resistant 2009 H1N1 in the U.S. Most cases were tested because drug resistance was suspected. All tested viruses retain their sensitivity to the neuraminidase inhibitor zanamivir. Of the 23 cases, 13 patients had documented exposure to oseltamivir through either treatment or chemoprophylaxis, nine patients are under investigation to determine exposure to oseltamivir, and one patient had no documented oseltamivir exposure. Occasional development of oseltamivir resistance during treatment or prophylaxis is not unexpected. Enhanced surveillance and increased availability of testing performed at CDC are expected to detect additional cases of oseltamivir resistant 2009 influenza A (H1N1) viruses, and such cases will be investigated to assess the spread of resistant strains in the community.
To prevent the spread of antiviral resistant virus strains, CDC reminds clinicians and the public of the need to continue hand and cough hygiene measures for the duration of any symptoms of influenza, even while taking antiviral medications (http://www.cy118119.com/mmwr/preview/mmwrhtml/mm5832a3.htm).
Pneumonia and Influenza (P&I) Mortality Surveillance
During week 46, 8.2% of all deaths reported through the 122-Cities Mortality Reporting System were due to P&I. This percentage was above the epidemic threshold of 7.0% for week 46. Including week 46, P&I mortality has been above threshold for eight consecutive weeks.
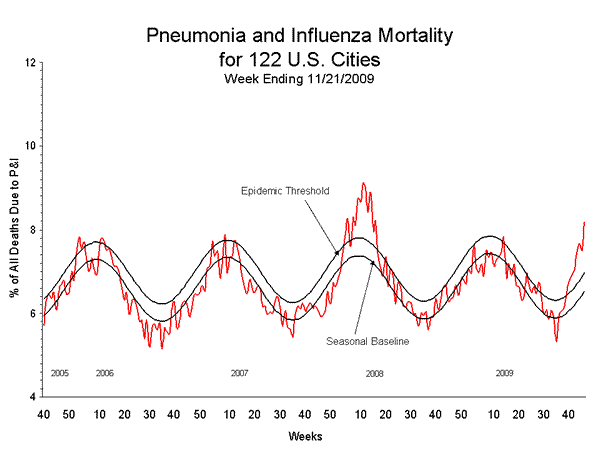
View Full Screen | View PowerPoint Presentation
Influenza-Associated Pediatric Mortality
Thirty-five influenza-associated pediatric deaths were reported to CDC during week 46 (California, Colorado, Florida [3], Illinois [3], Indiana, Kentucky, Massachusetts, Minnesota, Missouri, New Hampshire, New Mexico [8], New York, North Carolina [2], Pennsylvania [2], Rhode Island [2], South Carolina [2], Tennessee, Texas [2], and Washington). Twenty-seven of these deaths were associated with 2009 influenza A (H1N1) virus infection, seven were associated with an influenza A virus for which the subtype is undetermined, and one was associated with a seasonal influenza A (H1) virus infection. The deaths reported during week 46 occurred between March 8 and November 21, 2009.
One death associated with seasonal influenza A (H1) virus infection reported during week 46 occurred in March during the 2008-09 season, bringing the total number of reported pediatric deaths occurring during that season to 128.
Since August 30, 2009, CDC has received 172 reports of influenza-associated pediatric deaths that occurred during the current influenza season (30 deaths in children less than 2 years old, 18 deaths in children 2-4 years old, 65 deaths in children 5-11 years old, and 59 deaths in children 12-17 years old). One hundred forty (81%) of the172 deaths were due to 2009 influenza A (H1N1) virus infections, and the remaining 32 were associated with influenza A virus for which the subtype is undetermined. A total of 198 deaths in children associated with 2009 influenza A (H1N1) virus infection have been reported to CDC.
Among the 172 deaths in children, 84 children had specimens collected for bacterial culture from normally sterile sites and 26 (31.0%) of the 84 were positive; Staphylococcus aureus was identified in eight (30.8%) of the 26 children. One S. aureus isolate was sensitive to methicillin, six were methicillin resistant, and one did not have sensitivity testing performed. Seventeen (65.4%) of the 26 children with bacterial coinfections were five years of age or older, and seven (26.9%) of the 26 children were 12 years of age or older.
| Date | 2009 H1N1 Influenza | Influenza A-Subtype Unknown | Seasonal Influenza | Total |
|---|---|---|---|---|
| Number of Deaths REPORTED for Current Week – Week 46 (Week ending November 21, 2009) | 27 | 7 | 1 | 35 |
| Number of Deaths OCCURRED since August 30, 2009 | 140 | 32 | 0 | 172 |
| Number of Deaths OCCURRED since April 26, 2009 | 198 | 35 | 1 | 234 |
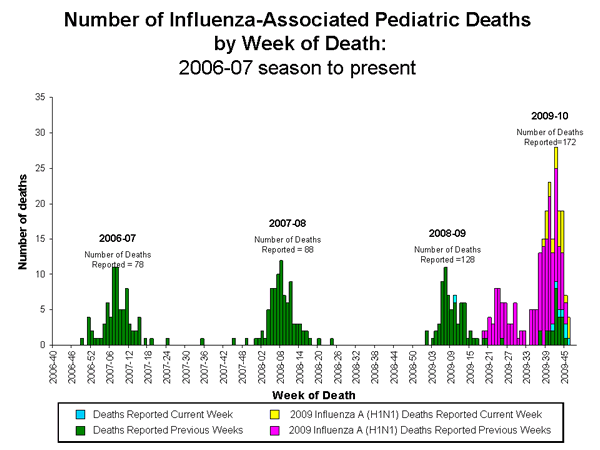
View Full Screen | View PowerPoint Presentation
Influenza-Associated Hospitalizations
Laboratory-confirmed influenza-associated hospitalizations are monitored using a population-based surveillance network that includes the 10 Emerging Infections Program (EIP) sites (CA, CO, CT, GA, MD, MN, NM, NY, OR and TN) and 6 new sites (IA, ID, MI, ND, OK and SD).
During September 1, 2009 – November 21, 2009, the following preliminary laboratory-confirmed overall influenza associated hospitalization rates were reported by EIP and the new sites (rates include influenza A, influenza B, and 2009 influenza A (H1N1)):
Rates [EIP (new sites)] for children aged 0-4 years and 5-17 years were 5.0 (8.9) and 2.3 (3.5) per 10,000, respectively. Rates [EIP (new sites)] for adults aged 18-49 years, 50-64 years, and ≥ 65 years were 1.9 (1.6), 2.4 (1.7) and 1.9 (1.5) per 10,000, respectively.
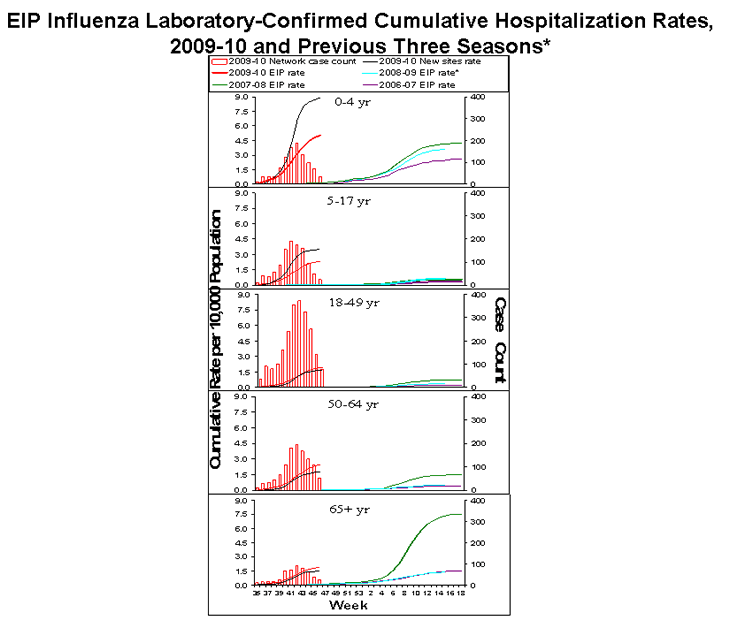
*The 2008-09 EIP rate ended as of April 14, 2009 due to the onset of the 2009 H1N1 season.
View Full Screen | View PowerPoint Presentation
Outpatient Illness Surveillance:
Nationwide during week 46, 4.3% of patient visits reported through the U.S. Outpatient Influenza-like Illness Surveillance Network (ILINet) were due to influenza-like illness (ILI). This percentage is above the national baseline of 2.3%.
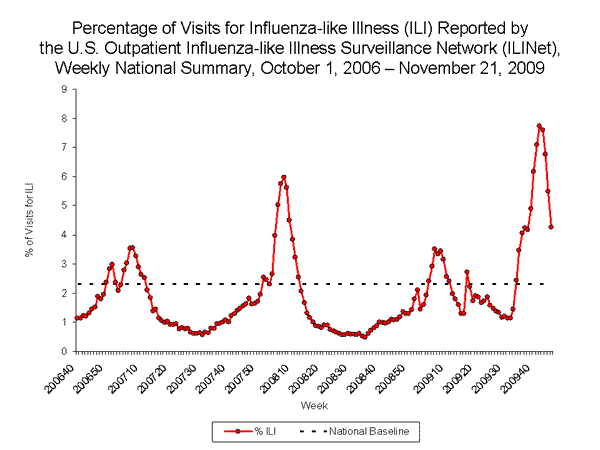
View ILINet Regional Charts | View Chart Data | View Full Screen | View PowerPoint Presentation
On a regional level, the percentage of outpatient visits for ILI ranged from 2.0% to 6.1% during week 46, and decreased in all 10 surveillance regions compared to the previous week. All 10 regions reported a proportion of outpatient visits for ILI above their region-specific baseline levels.
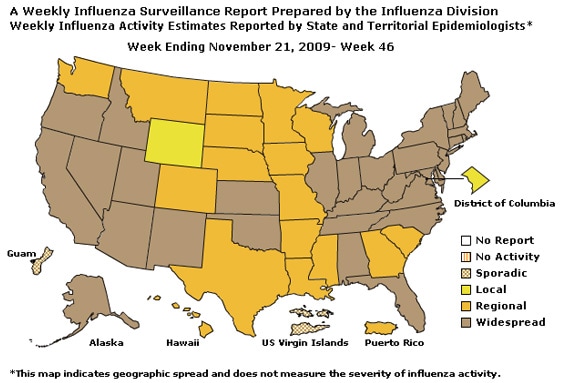
Geographic Spread of Influenza as Assessed by State and Territorial Epidemiologists:
The influenza activity reported by state and territorial epidemiologists indicates geographic spread of both seasonal influenza and 2009 influenza A (H1N1) viruses and does not measure the severity of influenza activity.
- During week 46, the following influenza activity was reported:
- Widespread influenza activity was reported by 32 states (Alabama, Alaska, Arizona, California, Connecticut, Delaware, Florida, Idaho, Illinois, Indiana, Kansas, Kentucky, Maine, Maryland, Massachusetts, Michigan, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, Tennessee, Utah, Vermont, Virginia, and West Virginia).
- Regional influenza activity was reported by Puerto Rico and 17 states (Arkansas, Colorado, Georgia, Hawaii, Iowa, Louisiana, Minnesota, Mississippi, Missouri, Montana, Nebraska, North Dakota, South Carolina, South Dakota, Texas, Washington, and Wisconsin).
- Local influenza activity was reported by the District of Columbia and one state (Wyoming).
- Sporadic influenza activity was reported by Guam and the U.S. Virgin Islands.
--------------------------------------------------------------------------------
A description of surveillance methods is available at: http://www.cy118119.com/flu/weekly/fluactivity.htm


