Error processing SSI file
Error processing SSI file
Weekly Report: Influenza Summary Update
Week ending October 8, 2005-Week 40
Error processing SSI fileBackground:
The Centers for Disease Control and Prevention (CDC) collects surveillance data year-round and reports on U.S. influenza activity each week from October through May. The U.S. influenza surveillance system consists of seven separate components: laboratory-based viral surveillance, mortality surveillance as reported through the 122 Cities Mortality Reporting System, influenza-associated pediatric mortality as reported through the Nationally Notifiable Disease Surveillance System, influenza-associated pediatric hospitalizations as reported through the Emerging Infections Programs in 10 sites and the New Vaccine Surveillance Network in 3 sites, sentinel provider surveillance for influenza-like illness (ILI), and state and territorial epidemiologist reports of influenza activity. This will be the second season that influenza-associated pediatric mortality data will be collected and the third season influenza-associated pediatric hospitalization data will be collected. Both surveillance components were initiated to estimate the impact of influenza on children.
These surveillance components enable CDC to determine when and where influenza activity is occurring, determine what types of influenza viruses are circulating, detect changes in the influenza viruses collected and analyzed, track patterns of influenza-related illness, and measure the impact of influenza in the United States. All influenza activity reporting by states, laboratories, and health-care providers is voluntary.
Synopsis:
During week 40 (October 2 - October 8, 2005)*, influenza activity occurred at a low level in the United States. Two (0.3%) specimens tested by U.S. World Health Organization (WHO) and National Respiratory and Enteric Virus Surveillance System (NREVSS) collaborating laboratories were positive for influenza. The proportion of patient visits to sentinel providers for influenza-like illness (ILI) and the proportion of deaths attributed to pneumonia and influenza were below baseline levels. Four states, New York City, and Puerto Rico reported sporadic influenza activity, and 44 states reported no influenza activity.
Laboratory Surveillance*:
About 80 WHO and 50 NREVSS collaborating laboratories located throughout the United States report the total number of respiratory specimens tested and the number positive for influenza types A and B each week. Some laboratories also report the subtype of the influenza A viruses (H1N1 or H3N2) they have isolated and the ages of the persons from whom the specimens were collected. A subset of the influenza viruses collected by laboratories are sent to CDC for more testing.
From week 21 through week 39 (weeks ending May 28 - October 1), WHO and NREVSS laboratories tested 18,126 specimens for influenza and 133 (0.7%) were positive. Isolates were reported from all surveillance regions** during the summer except the South Atlantic and East South Central regions. Eleven influenza A (H3N2) viruses, 2 influenza A (H1N1) viruses, and 10 influenza B viruses were collected during this period and antigenically characterized by CDC. Of the 11 influenza A (H3N2) viruses, 10 were characterized as A/California/7/2004-like, which is the (H3N2) recommended component for 2005-06 vaccine, and one showed somewhat reduced titers to ferret antisera produced against A/California/7/2004. Both influenza A (H1N1) viruses were characterized as A/New Caledonia/20/99-like, which is the (H1N1) component recommended for the vaccine.
During week 40, WHO and NREVSS laboratories reported 573 specimens tested for influenza viruses, and 2 were positive. Of the 2 influenza viruses identified, one was an influenza A (H3N2) virus from the Mid-Atlantic region and one was an influenza A virus that was not subtyped from the Pacific region.
Influenza B viruses circulating currently can be divided into two antigenically distinct lineages represented by B/Yamagata/16/88 and B/Victoria/2/87 viruses. B/Yamagata-like viruses have circulated widely since 1990. B/Victoria-like viruses had not been identified outside of Asia between 1991 and 2001. However, since March 2001, B/Victoria-like viruses have been identified in many countries, including the United States. Of the 10 influenza B viruses identified during May - September, 3 belonged to the B/Yamagata lineage and were characterized as B/Florida/07/2004-like, which is a minor antigenic variant of B/Shanghai/361/2002. A B/Shanghai/361/2002-like virus is the B component for the vaccine. The other 7 influenza B viruses belonged to the B/Victoria lineage.
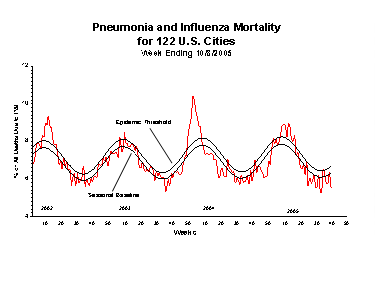
Pneumonia and Influenza (P&I) Mortality Surveillance*:
Each week, the vital statistics offices of 122 cities report the total number of death certificates filed and the number of those for which pneumonia or influenza was listed as the underlying or a contributing cause of death. The percentage of all deaths due to pneumonia and influenza are compared with baseline and epidemic threshold values calculated for each week.
During week 40, 5.5% of all deaths were reported as due to pneumonia or influenza. This percentage is below the epidemic threshold of 6.7% for week 40.
Influenza-Associated Pediatric Mortality:
Effective October 1, 2004, CDC has added influenza-associated pediatric (i.e., persons younger than 18 years of age) mortality to the list of nationally notifiable diseases voluntarily reportable through the National Notifiable Diseases Surveillance System (NNDSS). This action is based on recommendations developed collaboratively by the Council of State and Territorial Epidemiologists (CSTE) and CDC, and approved at the 2004 CSTE annual meeting. The recommended methods for surveillance are described in the 2004 CSTE position statement for influenza-associated pediatric mortality (www.cste.org). No influenza- associated pediatric deaths were reported for week 40.
Influenza-Associated Pediatric Hospitalizations:
Laboratory-confirmed influenza-associated pediatric hospitalizations are monitored in two population-based surveillance networks? the Emerging Infections Program (EIP) and the New Vaccine Surveillance Network (NVSN). EIP estimated rates of hospitalization for influenza will be reported every 2 weeks beginning October 28, 2005. NVSN estimated rates of hospitalization for influenza will be reported every 2 weeks, beginning November 25, 2005.
In years 2000-2005, the end-of-season hospitalization rate for NVSN ranged from 3.7 (2002-03) to 12 (2003-04) per 10,000 children. The 2003-04 end-of-season hospitalization rate for EIP was 8.9 per 10,000 children aged 0-4 years and 0.8 per 10,000 for children aged 5-17 years. The 2004-05 NVSN end-of-season hospitalization rate for children aged 0-4 years was 7 per 10,000. The preliminary 2004-05 end-of-season hospitalization rate for EIP was 3.3 per 10,000 children aged 0-4 years and 0.6 per 10,000 for children aged 5-17 years. The difference in rates between NVSN and EIP may be due to different case-finding methods and the different populations monitored. For a summary of the methods used in each system, please refer to the surveillance methods in the
Flu Activity section of the CDC influenza website.
Influenza-like Illness Surveillance*:
Each week, approximately 1,000 health-care providers around the country report the total number of patients seen and the number of those patients with influenza-like illness (ILI) by age group. For this system, ILI is defined as fever (temperature of >100癋) plus a cough and/or a sore throat, in the absence of a known cause other than influenza.
The percentage of patient visits to sentinel providers for ILI reported each week is weighted on the basis of state population. This percentage is compared each week with the national baseline of 2.2%. The baseline is the mean percentage of visits for ILI during non-influenza weeks for the previous 3 seasons plus 2 standard deviations.
During week 40, 1.0%*** of patient visits to U.S. sentinel providers were due to ILI. This percentage is less than the national baseline of 2.2%. On a regional level**, the percentage of visits for ILI ranged from 0.4% to 2.5%. Due to wide variability in regional level data, it is not appropriate to apply the national baseline to regional level data.
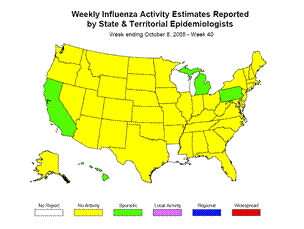
Influenza Activity as Assessed by State and Territorial Epidemiologists*:
Influenza activity was reported as sporadic in 4 states (California, Hawaii, Michigan, and Pennsylvania), New York City, and Puerto Rico. Forty-four states reported no influenza activity, and 2 states and the District of Columbia did not report.
--------------------------------------------------------------------------------
* Reporting is incomplete for this week. Numbers may change as more reports are received.
?/sup> The New Vaccine Surveillance Network (NVSN) conducts surveillance in Monroe County NY, Hamilton County OH, and Davidson County TN. The Emerging Infections Program (EIP) Influenza Project conducts surveillance in 57 counties associated with 11 metropolitan areas: San Francisco CA, Denver CO, New Haven CT, Atlanta GA, Baltimore MD, Minneapolis/St. Paul MN, Albuquerque NM, Albany NY, Rochester NY, Portland OR, and Nashville TN.
** Surveillance Regions: New England (Connecticut, Maine, Massachusetts, New Hampshire, Vermont, Rhode Island); Mid-Atlantic (New Jersey, New York City, Pennsylvania, Upstate New York); East North Central (Illinois, Indiana, Michigan, Ohio, Wisconsin); West North Central (Iowa, Kansas, Minnesota, Missouri, Nebraska, North Dakota, South Dakota); South Atlantic (Delaware, Florida, Georgia, Maryland, North Carolina, South Carolina, Virginia, Washington, D.C., West Virginia); East South Central (Alabama, Kentucky, Mississippi, Tennessee); West South Central (Arkansas, Louisiana, Oklahoma, Texas); Mountain (Arizona, Colorado, Idaho, Montana, Nevada, New Mexico, Utah, Wyoming); Pacific (Alaska, California, Hawaii, Oregon, Washington)
*** The national and regional percentage of patient visits for ILI is weighted on the basis of state population.
Report prepared October 14, 2005
Error processing SSI file