 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. 1997 USPHS/IDSA Guidelines for the Prevention of Opportunistic Infections in Persons Infected with Human Immunodeficiency VirusPREFACE In 1994, the U.S. Public Health Service (USPHS) and the Infectious Diseases Society of America (IDSA) recognized that, although strategies were available to reduce the frequency of opportunistic infections in patients who have human immunodeficiency virus (HIV) infection, information regarding prevention of both exposure and disease often was published in journals not regularly reviewed by health-care providers. In response, USPHS/IDSA developed comprehensive guidelines for health-care providers and patients that consolidated information pertaining to the prevention of opportunistic infections in persons infected with HIV. The resulting USPHS/IDSA guidelines were published in 1995 in the MMWR, Clinical Infectious Diseases, and the Annals of Internal Medicine, with an accompanying editorial in the Journal of the American Medical Association (1-4). The response to the 1995 guidelines (e.g., the many requests for reprints and observations from health-care providers) suggests that they have served as a valuable reference against which local policies regarding prevention of opportunistic infections could be compared. Because recommendations were rated on the basis of the strength of the evidence supporting them, readers were able to assess for themselves to which areas adherence was most important (5). In the United States, opportunistic infections continue to produce morbidity and mortality among the estimated 650,000-900,000 persons who are infected with HIV, especially among the estimated 200,000-250,000 persons who are severely immunosuppressed (i.e., persons who have a CD4+ T-lymphocyte count of less than 200 cells/uL) (6-10). However, surveillance data indicate that the incidence of opportunistic infections has been changing in the United States. In HIV-infected men who have sex with men, Pneumocystis carinii pneumonia (PCP), toxoplasmic encephalitis, fungal infections, and disseminated Mycobacterium avium complex (MAC) disease have decreased in incidence (9). Prophylactic regimens against opportunistic pathogens and more potent antiretroviral drugs appear to be important factors influencing this decline in incidence. However, these decreases have not been observed among HIV-infected injecting-drug users, suggesting that more emphasis should be placed on providing currently recommended chemoprophylactic agents to all persons who have HIV infection and who meet appropriate criteria for prophylaxis for opportunistic infections. The surveillance data also indicate that the incidence of some opportunistic infections is not decreasing among either men who have sex with men or injecting-drug users, indicating that preventive strategies need to be developed and applied to a wider spectrum of opportunistic infections. Because much new data concerning the prevention of opportunistic disease have emerged since 1994, the USPHS and the IDSA reconvened a working group on November 7-8, 1996, to determine which recommendations needed to be changed. Participants included representatives from federal agencies, universities, and professional societies, as well as community health-care providers and patient advocates. Most attention was focused on recent data related to chemoprophylaxis against disseminated MAC disease, cytomegalovirus (CMV), and fungal infections and to immunization against Streptococcus pneumoniae. However, data concerning all the common acquired immunodeficiency syndrome (AIDS)-associated pathogens were reviewed, as appropriate. Factors considered in revising guidelines included:
material presented at professional meetings. However, guidelines were revised only if complete manuscripts providing data were available for review. A review of the data that served as the basis for the revisions, as well as the additional information discussed at the meeting but not deemed appropriate to justify a revision of the recommendations, will be published elsewhere (11). The guidelines developed by the USPHS/IDSA working group were made available for public comment by an announcement in the Federal Register and in the MMWR, and the final document was approved by the USPHS and the IDSA, as well as by the American College of Physicians, the American Academy of Pediatrics, the Infectious Diseases Society of Obstetrics and Gynecology, the Society of Healthcare Epidemiologists of America, and the National Foundation for Infectious Diseases. How to Use the Information in This Report This report presents disease-specific recommendations for prevention of a) exposure to the opportunistic pathogen, b) first episode of disease, and c) disease recurrence, accompanied by a description of the rating system (see Box Table_B), categories of immunosuppression in HIV-infected children (Table_1), drugs and doses for prevention of first episode of disease and disease recurrence in adults (Table_2A and Table_2B) and children (Table_3A, Figure_1A, Figure_1B and Table_3B), recommendations for prevention of exposure to opportunistic pathogens (Table_4), and costs of commonly used prophylactic drugs and vaccines (Table_5). Because of their length and complexity, the tables and figure have been placed at the end of the text, preceding the references. Recommendations are rated by a revised version of the IDSA rating system (see Box Table_B) (5). In this system, a letter rating (letters A through E) signifies the strength of the recommendation; a Roman numeral (Roman numerals I through III) indicates the quality of the evidence supporting that recommendation. In the original guidelines (1-3), prophylaxis against PCP, toxoplasmic encephalitis, and tuberculosis for HIV-infected persons meeting appropriate criteria was rated A. In this updated report, the most important changes are a) the elevation from a B to an A rating of prophylaxis against disseminated MAC disease for both adults and children who have low CD4+ T-lymphocycte counts (Tables 2A and 3A), b) the recommendation that clarithromycin or azithromycin be considered first-choice drugs for MAC prophylaxis, with rifabutin as an alternative, and c) the elevation from a B to an A rating of vaccination against S. pneumoniae in adults with a CD4+ T-lymphocyte count greater than or equal to 200 cells/uL. The immunization schedule for HIV-infected children also has been revised to demonstrate the similarities and the differences between this schedule and that for immunocompetent children (Figure_1A Figure_1B). Prophylaxis against first episodes of CMV and fungal diseases remains optional, as in the earlier edition of the guidelines. Although not included in the disease-specific recommendations, an important issue in opportunistic-infection prophylaxis is whether to offer or continue prophylaxis on the basis of the lowest CD4+ T-lymphocyte count or of a more recent count that has been elevated as a result of antiretroviral therapy. This issue is particularly pertinent because of the administration of potent drug combinations that include protease inhibitors, which may increase CD4+ counts by 100-250 cells/uL. It is currently unknown whether such increases in CD4+ counts provide anti-infective protection comparable with that afforded to patients whose counts never declined below the current level. Until data assessing these risks are available, most experts recommend that prophylaxis be initiated or continued on the basis of the lowest CD4+ count. This report is oriented toward prevention of specific opportunistic infections in HIV-infected persons. Integrated approaches to preventing opportunistic infections in HIV-infected persons, as well as other aspects of HIV care, have been published elsewhere (12,13). Recommendations for antiretroviral therapy, which is designed to prevent immunologic deterioration and delay the need for many of the chemoprophylactic strategies described in this document, are also addressed separately (14). DISEASE-SPECIFIC RECOMMENDATIONS Pneumocystis carinii Pneumonia Prevention of Exposure (1) Although some authorities recommend that HIV-infected persons at risk for P. carinii pneumonia (PCP) not share a hospital room with a patient who has PCP, data are insufficient to support this recommendation as standard practice (CIII). Prevention of Disease (2) Adults and adolescents who have HIV infection (including those who are pregnant) should be administered chemoprophylaxis against PCP if they have a CD4+ T-lymphocyte count of less than 200/uL (AI), unexplained fever (greater than 100 F {37.7 C}) for greater than or equal to 2 weeks (AII), or a history of oropharyngeal candidiasis (AII). Trimethoprim-sulfamethoxazole (TMP-SMZ) is the preferred prophylactic agent (AI). One double-strength tablet/day is the preferred regimen (AI). However, one single-strength tablet/day also appears to be highly effective and may be better tolerated (AI). TMP-SMZ may confer cross-protection against toxoplasmosis (AII) and many bacterial infections (AII). For patients who have an adverse reaction that is not life-threatening, treatment with TMP-SMZ should be continued if clinically feasible; for those who have discontinued such therapy, its reinstitution should be strongly considered (AII). Whether it is best to reintroduce the drug at the original dose or at a lower and gradually increasing dose or to try a desensitization regimen is unknown. If TMP-SMZ cannot be tolerated, alternative prophylactic regimens include dapsone (BI), dapsone plus pyrimethamine plus leucovorin (BI), and aerosolized pentamidine administered by the Respirgard IITM nebulizer (Marquest, Englewood, CO) (BI). Regimens that include dapsone plus pyrimethamine also are protective against toxoplasmosis (AI) but not against most bacterial infections. Because data regarding their efficacy for PCP prophylaxis are insufficient for a firm recommendation, the following regimens generally cannot be recommended for this purpose: aerosolized pentamidine administered by other nebulization devices currently available in the United States, intermittently administered parenteral pentamidine, oral pyrimethamine/sulfadoxine, oral clindamycin plus primaquine, oral atovaquone, and intravenous trimetrexate. However, the use of these agents may be considered in unusual situations in which the recommended agents cannot be administered (CIII). Prevention of Recurrence (3) Adults and adolescents who have a history of PCP should be administered chemoprophylaxis with the regimens indicated above to prevent recurrence (AI). Notes Pediatric Notes (4) Children born to HIV-infected mothers should be administered prophylaxis with TMP-SMZ beginning at 4-6 weeks of age (15) (AII). Prophylaxis should be discontinued for children who are subsequently found not to be infected with HIV. HIV-infected children and children whose infection status remains unknown should continue to receive prophylaxis for the first year of life. The need for subsequent prophylaxis should be determined on the basis of age-specific CD4+ T-lymphocyte count thresholds (15,16, Table_1) (AII). (5) Children who have a history of PCP should be administered lifelong chemoprophylaxis to prevent recurrence (AI). Note Regarding Pregnancy (6) Chemoprophylaxis for PCP should be administered to pregnant women as well as to other adults and adolescents (AIII). TMP-SMZ is the recommended prophylactic agent. Because of theoretical concerns regarding possible teratogenicity associated with drug exposures during the first trimester, providers may choose to withhold prophylaxis with TMP-SMZ during the first trimester. In such cases, aerosolized pentamidine may be considered because of its lack of systemic absorption and the resultant lack of exposure of the developing embryo to the drug (CIII). Toxoplasmic Encephalitis Prevention of Exposure (1) HIV-infected persons should be tested for IgG antibody to Toxoplasma soon after the diagnosis of HIV infection to detect latent infection with Toxoplasma gondii (BIII). (2) All HIV-infected persons, but particularly those who lack IgG antibody to Toxoplasma, should be counseled about the various sources of toxoplasmic infection. They should be advised not to eat raw or undercooked meat, particularly undercooked pork, lamb, or venison (BIII). Specifically, meat should be cooked to an internal temperature of 150 F (65.5 C); meat cooked until it is no longer pink inside generally has an internal temperature of 165 F (73.8 C) and therefore satisfies this requirement. HIV-infected persons should wash their hands after contact with raw meat and after gardening or other contact with soil; in addition, they should wash fruits and vegetables well before eating them raw (BIII). If the patient owns a cat, the litter box should be changed daily, preferably by an HIV-negative, nonpregnant person; alternatively, the patient should wash the hands thoroughly after changing the litter box (BIII). Patients should be encouraged to keep their cats inside and not to adopt or handle stray cats (BIII). Cats should be fed only canned or dried commercial food or well-cooked table food, not raw or undercooked meats (BIII). Patients need not be advised to part with their cats or to have their cats tested for toxoplasmosis (EII). Prevention of Disease (3) Toxoplasma-seropositive patients who have a CD4+ T-lymphocyte count of less than 100/uL should be administered prophylaxis against toxoplasmic encephalitis (TE) (AII). The doses of TMP-SMZ recommended for PCP prophylaxis appear to be effective against TE as well (AII). If patients cannot tolerate TMP-SMZ, the regimens including dapsone plus pyrimethamine that are recommended for PCP prophylaxis provide protection against TE (AI). Prophylactic monotherapy with dapsone, pyrimethamine, azithromycin, clarithromycin, or atovaquone cannot be recommended on the basis of current data (DII). Aerosolized pentamidine does not afford protection against TE (EI). (4) Toxoplasma-seronegative persons who are not taking a PCP prophylactic regimen known to be active against TE should be retested for IgG antibody to Toxoplasma when their CD4+ T-lymphocyte count declines below 100/uL to determine whether they have seroconverted and are therefore at risk for TE (CIII). Patients who have seroconverted should be administered prophylaxis for TE as described above (AII). Prevention of Recurrence (5) Patients who have had TE should be administered lifelong suppressive therapy with drugs active against Toxoplasma to prevent relapse (AI). The combination of pyrimethamine plus sulfadiazine and leucovorin is highly effective for this purpose (AI). A commonly used regimen for patients who cannot tolerate sulfa drugs is pyrimethamine plus clindamycin (BI); however, only the combination of pyrimethamine plus sulfadiazine appears to provide protection against PCP as well (AII). Notes Pediatric Note (6) TMP/SMZ, when administered for PCP prophylaxis, also provides prophylaxis against toxoplasmosis. Children aged greater than 12 months who qualify for PCP prophylaxis and who are receiving an agent other than TMP/SMZ should have serologic testing for Toxoplasma antibody, because alternative drugs for PCP prophylaxis may not be effective against Toxoplasma (BIII). If seropositive for Toxoplasma, children should be administered prophylaxis for both PCP and toxoplasmosis (i.e, dapsone plus pyrimethamine)(BIII). Notes Regarding Pregnancy (7) TMP-SMZ can be administered for prophylaxis against TE as described for PCP (AIII). However, because of the low incidence of TE during pregnancy and the possible risk associated with pyrimethamine treatment, chemoprophylaxis with pyrimethamine-containing regimens can reasonably be deferred until after pregnancy (CIII). For prophylaxis against recurrent TE, the health-care provider and clinician should be well informed about the benefit of lifelong therapy and the concerns about teratogenicity of pyrimethamine. Most clinicians favor lifelong therapy for the mother, given the high likelihood that disease will recur promptly if therapy is stopped (AIII). (8) In rare cases, HIV-infected pregnant women who have serologic evidence of remote toxoplasmic infection have transmitted Toxoplasma to the fetus in utero. Pregnant HIV-infected women who have evidence of primary toxoplasmic infection or active toxoplasmosis (including TE) should be evaluated and managed during pregnancy in consultation with appropriate specialists (CIII). Infants born to women who have serologic evidence of infections with HIV and Toxoplasma should be evaluated for congenital toxoplasmosis (CIII). Cryptosporidiosis Prevention of Exposure (1) HIV-infected persons should be educated and counseled about the many ways that Cryptosporidium can be transmitted. Modes of transmission include contact with infected adults and diaper-aged children, contact with infected animals, drinking contaminated water, and contact with contaminated water during recreational activities (BIII). (2) HIV-infected persons should avoid contact with human and animal feces. They should be advised to wash their hands after contact with human feces (e.g., during diaper changing), after handling pets, and after gardening or other contact with soil. HIV-infected persons should avoid sexual practices that may result in oral exposure to feces (e.g., oral-anal intercourse) (BIII). (3) HIV-infected persons should be advised that newborn and very young pets may pose a small risk of cryptosporidial infection, but they should not be advised to destroy or give away healthy pets. Persons contemplating the acquisition of a new pet should avoid bringing any animal that has diarrhea into their households, should avoid purchasing a dog or cat aged less than 6 months and should not adopt stray pets. HIV-infected persons who wish to assume the small risk of acquiring a puppy or kitten aged less than 6 months should request that their veterinarian examine the animal's stool for Cryptosporidium before they have contact with the animal (BIII). (4) HIV-infected persons should avoid exposure to calves and lambs and to premises where these animals are raised (BII). (5) HIV-infected persons should not drink water directly from lakes or rivers (AIII). (6) Waterborne infection may also result from swallowing water during recreational activities. Patients should be aware that many lakes, rivers, and salt-water beaches and some swimming pools and recreational water parks may be contaminated with human or animal waste that contains Cryptosporidia. Patients should avoid swimming in water that is likely to be contaminated and should avoid swallowing water during swimming (BII). (7) Several outbreaks of cryptosporidiosis have been linked to municipal water supplies. During outbreaks or in other situations in which a community "boil-water" advisory is issued, boiling water for 1 minute will eliminate the risk of cryptosporidiosis (AI). Use of submicron personal-use water filters* (i.e., home/office types) and/or bottled water** may reduce the risk (CIII). The magnitude of the risk of acquiring cryptosporidiosis from drinking water in a nonoutbreak setting is uncertain, and current data are inadequate to recommend that all HIV-infected persons boil water or avoid drinking tap water in nonoutbreak settings. However, HIV-infected persons who wish to take independent action to reduce the risk of waterborne cryptosporidiosis may choose to take precautions similar to those recommended during outbreaks. Such decisions should be made in conjunction with health-care providers. Persons who opt for a personal-use filter or bottled water should be aware of the complexities involved in selecting appropriate products, the lack of enforceable standards for the destruction or removal of oocysts, the cost of the products, and the logistic difficulty of using these products consistently. (8) Patients who take precautions to avoid acquiring cryptosporidiosis from drinking water should be advised that ice made from contaminated tap water also can be a source of infection (BII). Such persons also should be aware that fountain beverages served in restaurants, bars, theaters, and other places may also pose a risk because these beverages, as well as the ice they contain, are made from tap water. Nationally distributed brands of bottled or canned carbonated soft drinks are safe to drink. Commercially packaged noncarbonated soft drinks and fruit juices that do not require refrigeration until after they are opened (e.g., those that can be stored unrefrigerated on grocery shelves) also are safe. Nationally distributed brands of frozen fruit juice concentrate are safe if they are reconstituted by the user with water from a safe source. Fruit juices that must be kept refrigerated from the time they are processed to the time of consumption may be either fresh (unpasteurized) or heat treated (pasteurized); only those juices labeled as pasteurized should be considered free of risk from Cryptosporidium. Other pasteurized beverages and beers also are considered safe to drink (BII). No data are available concerning survival of Cryptosporidium oocysts in wine. (9) In a hospital, standard precautions (i.e., use of gloves and hand washing after removal of gloves) should be sufficient to prevent transmission of cryptosporidiosis from an infected patient to a susceptible HIV-infected person (BII). However, because of the potential for fomite transmission, some experts recommend that HIV-infected persons, especially those who are severely immunocompromised, should not share a room with a patient with cryptosporidiosis (CIII). Prevention of Disease (10) No effective chemoprophylactic agents are available for cryptosporidiosis. Prevention of Recurrence (11) No drug regimens are known to be effective in preventing the recurrence of cryptosporidiosis. Note Pediatric Note (12) At present, no data indicate that formula-preparation practices for infants should be altered in an effort to prevent cryptosporidiosis (CIII). Microsporidiosis Prevention of Exposure (1) Other than general attention to hand washing and other personal hygiene measures, no precautions to reduce exposure can be recommended at this time. Prevention of Disease (2) No chemoprophylactic regimens are known to be effective in preventing microsporidiosis. Prevention of Recurrence (3) No chemotherapeutic regimens are known to be effective in preventing the recurrence of microsporidiosis. Tuberculosis Prevention of Exposure (1) HIV-infected persons should be advised that certain activities and occupations may increase the likelihood of exposure to tuberculosis (BIII). These include volunteer work or employment in health-care facilities, correctional institutions, and shelters for the homeless, as well as in other settings identified as high risk by local health authorities. Decisions about whether to continue with activities in these settings should be made in conjunction with the health-care provider and should be based on factors such as the patient's specific duties in the workplace, the prevalence of tuberculosis in the community, and the degree to which precautions are taken to prevent the transmission of tuberculosis in the workplace (BIII). Whether the patient continues with such activities may affect the frequency with which screening for tuberculosis needs to be conducted. Prevention of Disease (2) When HIV infection is first recognized, the patient should receive a tuberculin skin test (TST) by administration of intermediate-strength (5-TU) purified protein derivative (PPD) by the Mantoux method (AI). Routine evaluation for anergy is not recommended (CIII). However, there are selected situations in which anergy evaluation may assist in guiding individual decisions about preventive therapy (e.g., for TST-negative persons in populations at high risk for M. tuberculosis infection). (3) All HIV-infected persons who have a positive result in the TST (greater than or equal to 5 mm of induration) should undergo chest radiography and clinical evaluation for the exclusion of active tuberculosis. HIV-infected persons who have symptoms suggestive of tuberculosis should undergo chest radiography and clinical evaluation regardless of their TST status (AII). (4) All HIV-infected persons who have a positive TST result yet have no evidence of active tuberculosis and no history of treatment or prophylaxis for tuberculosis should be administered 12 months of preventive chemotherapy with isoniazid (INH) (AI). Because HIV-infected persons are at risk for peripheral neuropathy, those receiving INH should also receive pyridoxine (BIII). The decision to use alternative antimycobacterial agents for chemoprophylaxis should be based on the relative risk of exposure to resistant organisms and may require consultation with public health authorities (AII). Rifamycin/protease inhibitor interactions need to be taken into account when non-INH preventive therapy is considered. The need for direct observation as a means of documenting adherence to chemoprophylaxis should be considered on an individual basis (BIII). (5) HIV-infected persons who are close contacts of persons who have infectious tuberculosis should be administered preventive therapy -- regardless of TST results or prior courses of chemoprophylaxis -- after the diagnosis of active tuberculosis has been excluded (AII). In addition to household contacts, such persons might also include contacts in the same drug treatment or health-care facility, coworkers, and other contacts if transmission of TB is demonstrated. Such persons should be tested with 5-TU PPD. If the TST result is initially negative, the person should be evaluated again 3 months after the discontinuation of contact with the infectious source, and the information obtained should be considered in decisions about whether chemoprophylaxis should continue (BIII). (6) TST-negative, HIV-infected persons from risk groups or geographic areas with a high prevalence of M. tuberculosis infection may be at increased risk of primary or reactivation tuberculosis. Some experts recommend preventive therapy for some persons in this category (CIII). However, the efficacy of preventive therapy in this group has not been demonstrated, and such prophylaxis cannot be routinely recommended. Decisions concerning the use of chemoprophylaxis in these situations must be considered individually. (7) Although the reliability of the TST may diminish as the CD4+ T-lymphocyte count declines, annual repeat testing should be considered for HIV-infected persons who are TST-negative on initial evaluation and who belong to populations in which there is a substantial risk of exposure to M. tuberculosis (BIII). In addition to documenting tuberculous infection, TST conversion in an HIV-infected person should alert health-care providers to the possibility of recent M. tuberculosis transmission and should prompt notification of public health officials for investigation to identify a possible source case. (8) The administration of BCG vaccine to HIV-infected persons is contraindicated because of its potential to cause disseminated disease (EII). Prevention of Recurrence (9) Chronic suppressive therapy for a patient who has successfully completed a recommended regimen of treatment for tuberculosis is not necessary (DII). Notes Pediatric Note (10) Infants born to HIV-infected mothers should have a TST (5-TU PPD) at or before age 9-12 months and should be retested at least every 2-3 years (CIII). Children living in households with M. tuberculosis-infected (TST-positive) persons should be evaluated for tuberculosis (17) (CIII); children exposed to a person who has active tuberculosis should be administered preventive therapy after active tuberculosis has been excluded (AII). Decisions to discontinue prophylaxis for children who remain uninfected after removal from exposure to a source case can be made as for adults (see Prevention of Disease {5}). Note Regarding Pregnancy (11) Chemoprophylaxis for tuberculosis is recommended during pregnancy for HIV-infected patients who have either a positive TST or a history of exposure to active tuberculosis, after active tuberculosis has been excluded (AIII). A chest radiograph should be obtained before treatment and appropriate abdominal/pelvic lead apron shields should be used to minimize radiation exposure to the embryo/fetus. In the absence of exposure to drug-resistant TB, INH is the prophylactic agent of choice. Because of theoretical concerns regarding possible teratogenicity associated with drug exposures during the first trimester, providers may choose to initiate prophylaxis after the first trimester. Preventive therapy with INH should be accompanied by pyridoxine to reduce the risk of neurotoxicity. Experience with rifampin or rifabutin during pregnancy is more limited, but anecdotal experience with rifampin has not been associated with adverse pregnancy outcomes. Disseminated Infection with Mycobacterium avium Complex Prevention of Exposure (1) Organisms of the M. avium complex (MAC) are common in environmental sources such as food and water. Current information does not support specific recommendations regarding avoidance of exposure. Prevention of Disease (2) Adults and adolescents who have HIV infection should receive chemoprophylaxis against disseminated MAC disease if they have a CD4+ T-lymphocyte count of less than 50 cells/uL (AI). Clarithromycin or azithromycin are the preferred prophylactic agents (AI). The combination of clarithromycin and rifabutin is no more effective than clarithromycin alone for chemoprophylaxis and is associated with a higher rate of adverse effects than either drug alone; this combination should not be used (EI). The combination of azithromycin with rifabutin is more effective than azithromycin alone; however, the additional cost, increased occurrence of adverse effects, and absence of a difference in survival when compared with azithromycin alone do not warrant a routine recommendation for this regimen (CI). In addition to their preventive activity for MAC disease, clarithromycin and azithromycin confer protection against respiratory bacterial infections (BII). If clarithromycin or azithromycin cannot be tolerated, rifabutin is an alternative prophylactic agent for MAC disease (BI). Before prophylaxis is initiated, disseminated MAC disease should be ruled out by clinical assessment, which may include obtaining a blood culture for MAC if warranted. Because treatment with rifabutin could result in the development of resistance to rifampin in persons who have active tuberculosis, the latter condition should also be excluded before rifabutin is used for prophylaxis. Tolerance, cost, and drug interactions are among the issues that should be considered in decisions regarding the choice of prophylactic agents for MAC disease. Particular attention to interactions of antiretroviral protease inhibitors with rifabutin and, to a lesser extent, clarithromycin, is warranted (see Drug Interaction Note). (3) Although the detection of MAC organisms in the respiratory or gastrointestinal tract may be predictive of the development of disseminated MAC infection, no data are available on the efficacy of prophylaxis with clarithromycin, azithromycin, rifabutin, or other drugs in patients with MAC organisms at these sites and a negative blood culture. Therefore, routine screening of respiratory or gastrointestinal specimens for MAC cannot be recommended at this time (DIII). Prevention of Recurrence (4) Patients who are treated for disseminated MAC disease should continue to be administered full therapeutic doses of antimycobacterial agents for life (AII). The choice of the drug regimen should be made in consultation with an expert. Unless there is good clinical or laboratory evidence of macrolide resistance, the use of a macrolide (clarithromycin or azithromycin) is recommended in combination with at least one other drug (i.e., ethambutol {AII} or rifabutin {AII}). Treatment of MAC disease with clarithromycin in a dose of 1,000 mg twice a day is associated with decreased survival compared with clarithromycin administered 500 mg twice a day; thus, the higher dose should not be used (EI). Clofazimine has been demonstrated not to be effective in the treatment of MAC disease and should not be used (DII). Notes Drug Interaction Note (5) Patients concurrently being administered protease inhibitor antiretroviral therapy generally should not be administered rifabutin. However, if co-administration of rifabutin and a protease inhibitor is necessary, indinavir and nelfinavir are the preferred protease inhibitors, and the dose of rifabutin should be reduced by 50% with either of these drugs. Although protease inhibitors may also increase clarithromycin levels, no recommendation for dose adjustment of either clarithromycin or protease inhibitors can be made based on existing data. Pediatric Note (6) HIV-infected children aged less than 13 years who have advanced immunosuppression may also develop disseminated MAC infections, and prophylaxis should be offered to high-risk children according to the following CD4+ thresholds: children aged greater than or equal to 6 years, less than 50 cells/uL; children aged 2-6 years, less than 75 cells/uL; children aged 1-2 years, less than 500 cells/uL; and children aged less than 12 months, less than 750 cells/uL (AII). For the same reasons that clarithromycin and azithromycin are the preferred prophylactic agents for adults, they should also be considered for children (AII); oral suspensions of both are commercially available in the United States. A liquid formulation of rifabutin suitable for pediatric use is under development but currently is not commercially available in the United States. Note Regarding Pregnancy (7) Chemoprophylaxis for MAC disease should be administered to pregnant women as well as to other adults and adolescents (AIII). However, because of general concern about administering drugs during the first trimester of pregnancy, some providers may choose to withhold prophylaxis during the first trimester. Of the available agents, the safety profile in animal studies and anecdotal safety in humans suggest that azithromycin is the drug of choice (BIII). Experience with rifabutin is limited. Clarithromycin has been demonstrated to be a teratogen in animals and should be used with caution during pregnancy. Bacterial Respiratory Infections Prevention of Exposure (1) Because Streptococcus pneumoniae and Haemophilus influenzae are common in the community, there is no effective way to reduce exposure to these bacteria. Prevention of Disease (2) As soon as feasible after HIV infection is diagnosed, adults and adolescents who have a CD4+ T-lymphocyte count of greater than or equal to 200 cells/uL should be administered a single dose of 23-valent polysaccharide pneumococcal vaccine if they have not had this vaccine during the previous 5 years (AII). For persons who have a CD4+ T-lymphocyte count of less than 200 cells/uL, vaccination should be offered, although the humoral response and, therefore, clinical efficacy are likely to be diminished (CIII). The recommendation to vaccinate is increasingly pertinent because of the increasing incidence of invasive infections with drug-resistant (including TMP-SMZ-resistant and macrolide-resistant) strains of S. pneumoniae. Limited data suggest that administration of certain bacterial vaccines may transiently increase HIV replication and plasma HIV-1 RNA levels in HIV-infected persons who are not being administered potent antiretroviral regimens. However, evidence that adverse clinical outcomes are associated with this transient increase is lacking. Most experts believe that the benefit of pneumococcal vaccination outweighs the potential risk. (3) The duration of the protective effect afforded by primary pneumococcal vaccination is unknown. Revaccination once should be considered for HIV-infected persons, provided that at least 5 years have elapsed since administration of the first dose of pneumococcal vaccine (CIII). (4) The incidence of H. influenzae type B infection in adults is low. Therefore, H. influenzae type B vaccine is not generally recommended for adult use (DIII). (5) TMP-SMZ, administered daily, reduces the frequency of bacterial respiratory infections; this should be considered in the selection of an agent for PCP prophylaxis (AII). However, indiscriminate use of this drug (when not indicated for PCP prophylaxis or other specific reasons) may promote the development of TMP-SMZ-resistant organisms. Thus, TMP-SMZ should not be prescribed solely to prevent bacterial respiratory infection (DIII). Similarly, clarithromycin administered daily and azithromycin administered weekly are effective in preventing bacterial respiratory infections. This should be considered in the selection of an agent for prophylaxis of MAC disease (BII), although again, these drugs should not be prescribed solely for preventing bacterial respiratory infection (DIII). (6) An absolute neutrophil count that is depressed because of HIV disease or drug therapy may be increased by granulocyte colony-stimulating factor (G-CSF) or granulocyte-macrophage colony-stimulating factor (GM-CSF). The use of G-CSF or GM-CSF to prevent bacterial infections in HIV-infected patients with neutropenia cannot be recommended (CIII). However, preliminary data suggest that G-CSF can provide benefit for selected patients. Prevention of Recurrence (7) Some clinicians may administer antibiotic chemoprophylaxis to HIV-infected patients who have very frequent recurrences of serious bacterial respiratory infections (CIII). TMP-SMZ, administered for PCP prophylaxis, and clarithromycin or azithromycin, administered for MAC prophylaxis, are appropriate for drug-sensitive organisms. However, providers should be cautious about use of antibiotics for this purpose because of the potential for development of drug-resistant microorganisms. (8) All invasive pneumococcal isolates from HIV-infected patients should be tested for susceptibility to beta-lactam antibiotics, and local patterns of resistance should be considered in the choice of regimens for empirical treatment (AII). Invasive infections caused by H. influenzae should be treated with regimens effective against beta-lactamase-producing strains until drug susceptibilities are known (AII). Notes Pediatric Notes (9) Children who have HIV infection should be administered H. influenzae type b vaccine in accordance with the guidelines of the Advisory Committee on Immunization Practices (18) and the American Academy of Pediatrics (17) (AII). Children aged greater than 2 years also should be administered 23-valent polysaccharide pneumococcal vaccine (AII). Revaccination with pneumococcal vaccine generally should be offered after 3-5 years to children aged less than or equal to 10 years and after 5 years to children aged greater than 10 years (BIII). (10) To prevent serious bacterial infections in HIV-infected children who have hypogammaglobulinemia, clinicians should use intravenous immunoglobulin (IVIG) (AI). Respiratory syncytial virus (RSV) IVIG may be substituted for IVIG during the RSV season. (11) To prevent recurrence of serious bacterial respiratory infections, antibiotic chemoprophylaxis should be considered (BI). However, providers should be cautious about use of antibiotics for this purpose because of the potential for development of drug-resistant microorganisms. The administration of IVIG should also be considered for HIV-infected children who have recurrent serious bacterial infections (BI), but such treatment may not provide additional benefit to children who are being administered daily TMP-SMZ. Note Regarding Pregnancy (12) Pneumococcal vaccination is recommended during pregnancy for patients who have not been vaccinated during the previous 5 years (AIII). In nonpregnant adults, vaccination has been associated with a transient burst of HIV replication. It is unknown whether the transient viremia can increase the risk of perinatal HIV transmission. Because of this concern, when feasible, vaccination may be deferred until after antiretroviral therapy has been initiated for the prevention of perinatal HIV transmission (CIII). Bacterial Enteric Infections Prevention of Exposure Food (1) Health-care providers should advise HIV-infected persons not to eat raw or undercooked eggs (including foods that may contain raw eggs {e.g., some preparations of hollandaise sauce, Caesar and other salad dressings, and mayonnaise}); raw or undercooked poultry, meat, or seafood; or unpasteurized dairy products. Poultry and meat should be well cooked and should not be pink in the middle (internal temperature greater than 165 F {73.8 C}). Produce should be washed thoroughly before being eaten (BIII). (2) Health-care providers should advise HIV-infected persons to avoid cross-contamination of foods. For example, uncooked meats should not come into contact with other foods, and hands, cutting boards, counters, and knives and other utensils should be washed thoroughly after contact with uncooked foods (BIII). (3) Health-care providers should advise HIV-infected persons that, although the incidence of listeriosis is low, it is a serious disease that occurs with unusually high frequency among HIV-infected persons who are severely immunosuppressed. Such persons may choose to avoid soft cheeses because some studies have shown an association between these foods and listeriosis. These studies also have documented an association between ready-to-eat foods (e.g., hot dogs and cold cuts from delicatessen counters) and listeriosis. An immunosuppressed, HIV-infected person who wishes to reduce the risk of foodborne disease as much as possible may choose to reheat such foods until they are steaming hot before eating them (CIII). Pets (4) When obtaining a new pet, HIV-infected persons should avoid young animals (aged less than 6 months), especially those that have diarrhea (BIII). (5) HIV-infected persons should avoid contact with animals that have diarrhea (BIII). HIV-infected pet owners should seek veterinary care for animals with diarrheal illness, and a fecal sample from such animals should be examined for Cryptosporidium, Salmonella, and Campylobacter. (6) HIV-infected persons should wash their hands after handling pets (especially before eating) and should avoid contact with pets' feces (BIII). (7) HIV-infected persons should avoid contact with reptiles (e.g., snakes, lizards, iguanas, and turtles) because of the risk of salmonellosis (BIII). Travel (8) The risk of food- and waterborne infections among immunosuppressed, HIV-infected persons is magnified during travel to developing countries. Those who elect to travel to such countries should avoid foods and beverages that may be contaminated, particularly raw fruits and vegetables, raw or undercooked seafood or meat, tap water, ice made with tap water, unpasteurized milk and dairy products, and items sold by street vendors (AII). Foods and beverages that are generally safe include steaming-hot foods, fruits that are peeled by the traveler, bottled (especially carbonated) beverages, hot coffee and tea, beer, wine, and water brought to a rolling boil for 1 minute (AII). Treatment of water with iodine or chlorine may not be as effective as boiling but can be used when boiling is not practical (BIII). Prevention of Disease (9) Prophylactic antimicrobial agents are not generally recommended for travelers (DIII). The effectiveness of these agents depends on local antimicrobial-resistance patterns of gastrointestinal pathogens, which are seldom known. Moreover, these agents can elicit adverse reactions and can promote the emergence of resistant organisms. However, for HIV-infected travelers, antimicrobial prophylaxis may be considered, depending on the level of immunosuppression and the region and duration of travel (CIII). The use of fluoroquinolones such as ciprofloxacin (500 mg/d) can be considered when prophylaxis is deemed necessary (BIII). As an alternative (e.g., for children, pregnant women, and persons already taking TMP-SMZ for PCP prophylaxis), TMP-SMZ may offer some protection against traveler's diarrhea (BIII). The risk of toxicity should be considered before treatment with TMP-SMZ is initiated solely because of travel. (10) Antimicrobial agents such as fluoroquinolones (e.g., 500 mg of ciprofloxacin twice a day for 3-7 days) should be given to patients before their departure, to be taken empirically should traveler's diarrhea develop (BIII). Alternative antibiotics for children and pregnant women should be discussed (CIII). Travelers should consult a physician if their diarrhea is severe and does not respond to empirical therapy, if their stools contain blood, if fever is accompanied by shaking chills, or if dehydration develops. Antiperistaltic agents (e.g., diphenoxylate and loperamide) can be used for the treatment of mild diarrhea. However, the use of these drugs should be discontinued if symptoms persist beyond 48 hours. Moreover, these agents should not be administered to patients who have a high fever or who have blood in the stool (AII). (11) Some experts recommend that HIV-infected persons who have Salmonella gastroenteritis be administered antimicrobial therapy to prevent extraintestinal spread. However, no controlled study has demonstrated a beneficial effect of such treatment, and some studies of immunocompetent persons have suggested that antimicrobial therapy can lengthen the shedding period. The fluoroquinolones -- primarily ciprofloxacin (750 mg twice a day for 14 days) -- can be used when antimicrobial therapy is chosen (CIII). Prevention of Recurrence (12) HIV-infected persons who have Salmonella septicemia require long-term therapy for the prevention of recurrence. The fluoroquinolones, primarily ciprofloxacin, are usually the drugs of choice for susceptible organisms (BII). (13) Household contacts of HIV-infected persons who have salmonellosis or shigellosis should be evaluated for asymptomatic carriage of Salmonella or Shigella so that strict hygienic measures and/or antimicrobial therapy can be instituted and recurrent transmission to the HIV-infected person can be prevented (CIII). Notes Pediatric Notes (14) Like HIV-infected adults, HIV-infected children should wash their hands after handling pets (especially before eating) and should avoid contact with pets' feces. Hand washing should be supervised (BIII). (15) HIV-exposed infants aged less than 3 months and all HIV-infected children who have severe immunosuppression should be administered treatment for Salmonella gastroenteritis to prevent extraintestinal spread (CIII). Possible choices of antibiotics include TMP-SMZ, ampicillin, cefotaxime, ceftriaxone, or chloramphenicol; ciprofloxacin should be used with caution and only if no alternatives exist. (16) HIV-infected children who have Salmonella septicemia should be offered long-term therapy for the prevention of recurrence (CIII). TMP-SMZ is the drug of choice; ampicillin or chloramphenicol can be used if the organism is susceptible. Ciprofloxacin should be used with caution and only if no alternatives exist. (17) Antiperistaltic drugs are not recommended for children (DIII). Notes Regarding Pregnancy (18) Because both pregnancy and HIV infection confer a risk for listeriosis, pregnant HIV-infected women should heed recommendations concerned with this disease (BII). (19) Fluoroquinolones should not be used during pregnancy. TMP-SMZ may offer some protection against traveler's diarrhea. Infection with Bartonella (Formerly Rochalimaea) Prevention of Exposure (1) HIV-infected persons, particularly those who are severely immunosuppressed, are at unusually high risk of developing relatively severe disease due to Bartonella species. These persons should consider the potential risks of cat ownership (CIII). Those who elect to acquire a cat should adopt or purchase an older animal (aged greater than 1 year) that is in good health (BII). (2) Although declawing is not generally advised, HIV-infected persons should avoid rough play with cats and situations in which scratches are likely (BII). Any cat-associated wound should be washed promptly (CIII). HIV-infected persons should not allow cats to lick open cuts or wounds (BIII). (3) Care of cats should include flea control (CIII). (4) There is no evidence of benefit to cat or owner from routine culture or serologic testing of the pet for Bartonella infection (DII). Prevention of Disease (5) No data currently support chemoprophylaxis for Bartonella-associated disease (CIII). Prevention of Recurrence (6) Relapse or reinfection with Bartonella has sometimes followed a course of primary treatment. Although no firm recommendation can be made regarding prophylaxis in this situation, long-term suppression of infection with erythromycin or doxycycline should be considered (CIII). Note Pediatric Note (7) The risks of cat ownership for HIV-infected children who are severely immunocompromised should be discussed with parents/caretakers (CIII). Candidiasis Prevention of Exposure (1) Candida organisms are common on mucosal surfaces and skin. No measures are available to reduce exposure to these fungi. Prevention of Disease (2) Data from a prospective controlled trial indicate that fluconazole can reduce the risk of mucosal (oropharyngeal, esophageal, and vaginal) candidiasis (and cryptococcosis as well) in patients with advanced HIV disease. However, routine primary prophylaxis is not recommended because of the effectiveness of therapy for acute disease, the low mortality associated with mucosal candidiasis, the potential for resistant Candida organisms to develop, the possibility of drug interactions, and the cost of prophylaxis (CI). Prevention of Recurrence (3) Many experts do not recommend chronic prophylaxis of recurrent oropharyngeal or vulvovaginal candidiasis for the same reasons that they do not recommend primary prophylaxis. However, if recurrences are frequent or severe, intermittent or chronic administration of an oral azole (fluconazole {AI}, ketoconazole {CIII}, or itraconazole {CIII}) may be considered. Other factors that influence choices about such therapy include the impact of the recurrences on the patient's well-being and quality of life, the need for prophylaxis for other fungal infections, cost, toxicities, and drug interactions. (4) Adults or adolescents who have a history of documented esophageal candidiasis, particularly multiple episodes, should be considered candidates for chronic suppressive therapy with fluconazole (BI). Notes Pediatric Notes (5) Primary prophylaxis of candidiasis in HIV-infected infants is not indicated (DIII). (6) Suppressive therapy with systemic azoles should be considered for infants who have severe recurrent mucocutaneous candidiasis (BIII) and particularly for those who have esophageal candidiasis (BI). Note Regarding Pregnancy (7) There is limited experience with the chronic use of antimycotic drugs during human pregnancy; three cases of infants born with craniofacial and skeletal abnormalities following prolonged in-utero exosure to fluconazole have been reported. Therefore, chemoprophylaxis against oropharyngeal, esophageal, or vaginal candidiasis should not be initiated during pregnancy (DIII). The drug should be discontinued for patients who conceive while being administered the drug. Cryptococcosis Prevention of Exposure (1) Although HIV-infected persons cannot completely avoid exposure to Cryptococcus neoformans, avoiding sites that are likely to be heavily contaminated with C. neoformans (e.g., areas heavily contaminated with pigeon droppings) may reduce the risk of infection. Prevention of Disease (2) Routine testing of asymptomatic persons for serum cryptococcal antigen is not recommended because of the low probability that the results will affect clinical decisions (DIII). (3) Data from prospective controlled trials indicate that fluconazole and itraconazole can reduce the frequency of cryptococcal disease among patients who have advanced HIV disease. Therefore, providers may wish to consider prophylaxis for persons who have a CD4+ T-lymphocyte count of less than 50 cells/uL (CI). However, most experts recommend that antifungal prophylaxis not be used routinely to prevent cryptococcosis because of the relative infrequency of cryptococcal disease, the lack of survival benefit associated with prophylaxis, the possibility of drug interactions, the potential for development of both Candida and Cryptococcus drug resistance, and cost. The need for prophylaxis or suppressive therapy for other fungal infections (e.g., candidiasis or histoplasmosis) should be considered in making decisions about prophylaxis for cryptococcosis. Doses of fluconazole ranging from 400 mg once a week to 200 mg daily are effective as prophylaxis against cryptococcosis; however, doses of less than 200 mg daily may be less effective in suppressing Candida infections, and fluconazole may not prevent Histoplasma infection. Prevention of Recurrence (4) Patients who complete initial therapy for cryptococcosis should be administered lifelong suppressive treatment with fluconazole (AI). Notes Pediatric Note (5) There are no data on which to base specific recommendations for children, but lifelong suppressive therapy with fluconazole after an episode of cryptococcosis is appropriate (AII). Note Regarding Pregnancy (6) Prophylaxis with fluconazole or itraconazole should not be initiated during pregnancy because of the low incidence of cryptococcal disease, the lack of a recommendation for primary prophylaxis against cryptococcosis in nonpregnant adults, and the potential for adverse effects of these drugs during pregnancy (DIII). For patients who conceive while being administered primary prophylaxis, prophylaxis should be discontinued. However, because of the risk of the disease to maternal health, prophylaxis against recurrent cryptococcal disease with fluconazole during pregnancy is indicated (AIII). Histoplasmosis Prevention of Exposure (1) Although HIV-infected persons living in or visiting histoplasmosis-endemic areas cannot completely avoid exposure to Histoplasma capsulatum, they should avoid activities known to be associated with increased risk (e.g., cleaning chicken coops, disturbing soil beneath bird-roosting sites, and exploring caves) (CIII). Prevention of Disease (2) Routine skin testing with histoplasmin in histoplasmosis-endemic areas is not predictive of disease and should not be performed (EII). (3) Data from a prospective controlled trial indicate that itraconazole can reduce the frequency of histoplasmosis among patients who have advanced HIV infection and who live in areas in which H. capsulatum is endemic; thus, physicians may wish to consider chemoprophylaxis for adult and adolescent patients who live in an area endemic for H. capsulatum and who have a CD4+ T-lymphocyte count of less than 100 cells/uL (CI). However, when deciding on such prophylaxis, physicians should consider the possibility of drug interactions, toxicity, development of resistance, and the cost of prophylaxis. The need for prophylaxis or suppressive therapy for other fungal infections (e.g., cryptococcosis and candidiasis) should be considered when making decisions about prophylaxis for histoplasmosis. Itraconazole has not been demonstrated conclusively to prevent candidiasis, although it has activity for chronic suppression of cryptococcosis. Prevention of Recurrence (4) Patients who complete initial therapy should be administered lifelong suppressive treatment with itraconazole (AII). Notes Pediatric Note (5) Because primary histoplasmosis can lead to disseminated infection in children, it is reasonable to administer lifelong suppressive therapy after an acute episode of the disease (AIII). Note Regarding Pregnancy (6) Itraconazole is embryotoxic and teratogenic in animal systems. Therefore, primary prophylaxis against histoplasmosis is not indicated during pregnancy (DIII). However, because of the risk of the disease to maternal health, prophylaxis against recurrent histoplasmosis is indicated (AIII). Coccidioidomycosis Prevention of Exposure (1) Although HIV-infected persons living in or visiting areas in which coccidioidomycosis is endemic cannot completely avoid exposure to Coccidioides immitis, they should, when possible, avoid activities associated with increased risk (e.g., those involving extensive exposure to disturbed native soil, for example, at building excavation sites or during dust storms) (CIII). (2) Routine skin testing with coccidioidin (spherulin) in coccidioidomycosis-endemic areas is not predictive of disease and should not be performed (EII). (3) No recommendation can be made regarding routine chemoprophylaxis for HIV-infected persons who live in coccidioidomycosis-endemic areas or for skin test-positive persons who live in areas where coccidioidomycosis is not endemic. Prevention of Recurrence (4) Patients who complete initial therapy for coccidioidomycosis should be administered lifelong systemic suppressive treatment (AII). Fluconazole is the preferred agent; alternative drugs include itraconazole and amphotericin B. Notes Pediatric Note (5) Although no specific data are available regarding coccidioidomycosis in HIV-infected children, it is reasonable to administer lifelong suppressive therapy after an acute episode of the disease (AIII). Note Regarding Pregnancy (6) Because of the risk to maternal health posed by coccidioidomycosis, prophylaxis against recurrent coccidioidomycosis is indicated during pregnancy (AIII). Cytomegalovirus Disease Prevention of Exposure (1) HIV-infected persons who belong to risk groups with relatively low rates of seropositivity for cytomegalovirus (CMV) and who anticipate possible exposure to CMV (e.g., through blood transfusion or employment in a child-care facility) should be tested for antibody to CMV (BIII). These groups include patients who have not had male homosexual contact and those who are not injecting-drug users. (2) HIV-infected adolescents and adults should be advised that CMV is shed in semen, cervical secretions, and saliva and that latex condoms must always be used during sexual contact to reduce the risk of exposure to CMV and to other sexually transmitted pathogens (AII). (3) HIV-infected adults and adolescents who are child-care providers or parents of children in child-care facilities should be informed that they--like all children at these facilities--are at increased risk of acquiring CMV infection (BI). Parents and other caretakers of HIV-infected children should be advised of the increased risk to children at these centers (BIII). The risk of acquiring CMV infection can be diminished by good hygienic practices such as hand washing (AII). (4) HIV-exposed infants and HIV-infected children, adolescents, and adults who are seronegative for CMV and require blood transfusion should be administered only CMV antibody-negative or leukocyte-reduced cellular blood products in nonemergency situations (BIII). Prevention of Disease (5) Prophylaxis with oral ganciclovir may be considered for HIV-infected adults and adolescents who are CMV seropositive and who have a CD4+ T-lymphocyte count of less than 50 cells/uL (CI). Neutropenia, anemia, limited efficacy, lack of improvement in survival, and cost are among the issues that should be considered in decisions about whether to institute prophylaxis in individual patients. Acyclovir is not effective in preventing CMV disease, and valaciclovir is not recommended because of an unexplained trend toward increased mortality observed in persons who have AIDS and who were administered this drug for CMV prophylaxis. Therefore, neither acyclovir nor valaciclovir should be used for this purpose (EI). The most important method for preventing severe CMV disease is recognition of the early manifestations of the disease. Early recognition of CMV retinitis is most likely when the patient has been educated on this topic. Patients should be made aware of the significance of increased "floaters" in the eye and should be advised to assess their visual acuity regularly by simple techniques such as reading newsprint (BIII). Regular funduscopic examinations performed by a health-care provider or specifically by an ophthalmologist are recommended by some experts for patients with low (e.g., less than 100 cells/uL) CD4+ T-lymphocyte counts (CIII). Prevention of Recurrence (6) CMV disease is not cured with courses of the currently available antiviral agents (i.e., ganciclovir, foscarnet, or cidofovir). Chronic suppressive or maintenance therapy is indicated. Effective regimens include parenteral or oral ganciclovir, parenteral foscarnet, combined parenteral ganciclovir and foscarnet, parenteral cidofovir, and (for retinitis only) ganciclovir administration via intraocular implant (AI). The intraocular implant does not provide protection to the contralateral eye or to other organ systems. In spite of maintenance therapy, recurrences develop routinely and require reinstitution of high-dose induction therapy or replacement of the implant. Notes Pediatric Note (7) Some experts recommend obtaining a CMV urine culture on all HIV-infected (or exposed) infants at birth or at an early postnatal visit to identify those infants with congenital CMV infection (CIII). In addition, beginning at 1 year of age, CMV antibody testing on an annual basis may be considered for CMV-seronegative (and culture-negative) HIV-infected infants and children who are severely immunosuppressed (Table_1) (CIII). Annual testing will allow identification of children who have acquired CMV infection and might benefit from screening for retinitis. (8) HIV-infected children who are CMV-infected and severely immunosuppressed may benefit from a dilated retinal examination performed by an ophthalmologist every 4-6 months (CIII). In addition, older children should be counseled to be aware of "floaters" in the eye, similar to the recommendation for adults (BIII). (9) Oral ganciclovir is currently under investigation in CMV-infected children, and no recommendation about its use can be made at this time. Note Regarding Pregnancy (10) Because of the lack of recommendation for its routine use in nonpregnant adults and the lack of experience with this drug during pregnancy, ganciclovir is not recommended for primary prophylaxis against CMV disease during pregnancy (DIII). Ganciclovir should be discontinued for patients who conceive while being administered primary prophylaxis. Because of the risks to maternal health, prophylaxis against recurrent CMV disease is indicated during pregnancy (AIII). The choice of agents to be used in pregnancy should be individualized after consultation with experts. Herpes Simplex Virus Disease Prevention of Exposure (1) HIV-infected persons should use latex condoms during every act of sexual intercourse to reduce the risk of exposure to herpes simplex virus (HSV) and to other sexually transmitted pathogens (AII). They should specifically avoid sexual contact when herpetic lesions (genital or orolabial) are evident (AII). Prevention of Disease (2) Prophylaxis of initial episodes of HSV disease is not recommended (DIII). Prevention of Recurrence (3) Because acute episodes of HSV infection can be treated successfully, chronic therapy with acyclovir is not required after lesions resolve. However, persons who have frequent or severe recurrences can be administered daily suppressive therapy with oral acyclovir (AI). Intravenous foscarnet or cidofovir can be used to treat infection due to acyclovir-resistant isolates of HSV, which are routinely resistant to ganciclovir as well (AII). Topical preparations of foscarnet and cidofovir also are available. Notes Pediatric Note (4) The recommendations for the prevention of initial disease and recurrence apply to children as well as to adolescents and adults. Note Regarding Pregnancy (5) Oral acyclovir prophylaxis in late pregnancy is a controversial strategy recommended by some experts to prevent neonatal herpes transmission. However, such prophylaxis is not routinely recommended. For patients who have frequent, severe recurrences of genital HSV disease, acyclovir prophylaxis may be indicated (BIII). No pattern of adverse pregnancy outcomes has been reported after acyclovir exposures. Varicella-Zoster Virus Infection Prevention of Exposure (1) HIV-infected children and adults who are susceptible to varicella-zoster virus (VZV) (i.e., those who have no history of chickenpox or shingles or are seronegative for VZV) should avoid exposure to persons with chickenpox or shingles (AII). Although vaccination against varicella is currently under investigation in HIV-infected children, based on current information, vaccine against varicella should not be administered to adults or children who are infected with HIV because of the potential for disseminated viral infection (EIII). Household contacts (especially children) of susceptible HIV-infected persons should be vaccinated against VZV if they have no history of chickenpox and are seronegative for HIV, so that they will not transmit VZV to their susceptible HIV-infected contact (BIII). Prevention of Disease (2) For the prophylaxis of chickenpox, HIV-infected children and adults who are susceptible to VZV (i.e., those who have no history of chickenpox or shingles or who have no detectable antibody against VZV) should be administered varicella zoster immune globulin (VZIG) as soon as possible but within 96 hours after close contact with a patient who has chickenpox or shingles (AIII). Data are lacking on the effectiveness of acyclovir for preventing chickenpox in susceptible HIV-infected children or adults, although such an approach would be logical (CIII). (3) No preventive measures are currently available for shingles. Prevention of Recurrence (4) No drug has been proven to prevent recurrence of shingles in HIV-infected persons. Note Note Regarding Pregnancy (5) VZIG is recommended for VZV-susceptible pregnant women within 96 hours after exposure to VZV (AIII). If oral acyclovir is used, VZV serology should be performed so that the drug can be discontinued if the patient is seropositive for VZV (BIII). Human Papillomavirus Infection Prevention of Exposure (1) HIV-infected persons should use latex condoms during every act of sexual intercourse to reduce the risk of exposure to human papillomavirus (HPV) as well as to other sexually transmitted pathogens (AIII). Prevention of Disease HPV-associated genital epithelial cancers in HIV-infected women (2) After a complete history of previous cervical disease has been obtained, HIV-infected women should have a pelvic examination and a Pap smear. In accordance with the recommendation of the Agency for Health Care Policy and Research (13), the Pap smear should be obtained twice in the first year after diagnosis of HIV infection and, if the results are normal, annually thereafter (AII). (3) If the results of the Pap smear are abnormal, care should be provided according to the Interim Guidelines for Management of Abnormal Cervical Cytology published by a National Cancer Institute Consensus Panel (19) and briefly summarized below. (4) For patients whose Pap smears are interpreted as atypical squamous cells of undetermined significance (ASCUS), several management options are available; the choice depends in part on whether the interpretation of ASCUS is qualified by a statement indicating that a neoplastic process is favored. Follow-up by Pap tests without colposcopy is acceptable, particularly when the diagnosis of ASCUS is not qualified further or the cytopathologist favors a reactive process. In such situations, Pap tests should be repeated every 4-6 months for 2 years until three consecutive smears have been negative. If a second report of ASCUS occurs in the 2-year follow-up period, the patient should be considered for colposcopic evaluation (BIII). Women who have a diagnosis of unqualified ASCUS associated with severe inflammation should be evaluated for an infectious process. If specific infections are identified, reevaluation should be performed after appropriate treatment, preferably after 2-3 months (BIII). If the diagnosis of ASCUS is qualified by a statement indicating that a neoplastic process is favored, the patient should be managed as if a low-grade squamous intraepithelial lesion (LSIL) were present (see note {5}) (BIII). If a patient who has a diagnosis of ASCUS is at high risk (i.e., previous positive Pap tests or poor compliance with follow-up), the option of colposcopy should be considered (BIII). (5) Several management options are available for patients who have LSIL. Follow-up with Pap tests every 4-6 months is used by many clinicians and is currently used in countries outside the United States as an established method of management. Patients managed in this fashion must be carefully selected and considered reliable for follow-up. If repeat smears show persistent abnormalities, colposcopy and directed biopsy are indicated (BIII). Colposcopy and directed biopsy of any abnormal area on the ectocervix constitute another appropriate option (BIII). (6) Women who have cytologic diagnosis of high-grade squamous intraepithelial lesions (HSIL) or squamous cell carcinoma should undergo colposcopy and directed biopsy (AII). HPV-associated anal intraepithelial neoplasia and anal cancer in HIV-infected men who have sex with men (7) Although the risks for anal intraepithelial neoplasia (AIN) and anal cancer are increased among HIV-infected men who have sex with men, the role of anal cytologic screening and treatment of AIN in preventing anal cancer in these men is not well defined. Therefore, no recommendations can be made for periodic anal cytologic screening for the detection and treatment of AIN. Prevention of Recurrence (8) The risks for recurrence of squamous intraepithelial lesions and cervical cancer after conventional therapy are increased among HIV-infected women. The prevention of illness associated with recurrence depends on careful follow-up of patients after treatment. Patients should be monitored with frequent cytologic screening and, when indicated, with colposcopic examination for recurrent lesions (AI).
References
Table_B Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
CATEGORIES REFLECTING STRENGTH AND QUALITY OF EVIDENCE
Rating system for strength of recommendation and quality of
evidence supporting the recommendation *
A Both strong evidence for efficacy and substantial clinical
benefit support recommendation for use. Should always be offered.
B Moderate evidence for efficacy -- or strong evidence for
efficacy,, but only limited clinical benefit -- supports
recommendation for use. Should generally be offered.
C Evidence for efficacy is insufficient to support a
recommendation for or against use,, or evidence for efficacy may
not outweigh adverse consequences (e.g.,, toxicity,, drug
interactions,, or cost of the chemoprophylaxis or alternative
approaches). Optional.
D Moderate evidence for lack of efficacy or for adverse outcome
supports a recommendation against use. Should generally not be
offered.
E Good evidence for lack of efficacy or for adverse outcome
supports a recommendation against use. Should never be offered.
Categories Reflecting Quality of Evidence Supporting the
Recommendation
I Evidence from at least one properly randomized,, controlled
trial
II Evidence from at least one well-designed clinical trial
without randomization,, from cohort or case-controlled analytic
studies (preferably from more than one center),, or from multiple
time-series studies or dramatic results from uncontrolled
experiments
III Evidence from opinions of respected authorities based on
clinical experience,, descriptive studies,, or reports of expert
committees
* Modified from (5).
Return to top. Table_1 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 1. Immunologic categories for HIV-infected children based on age-specific CD4+
T-lymphocyte counts and percentage of total lymphocytes *
===================================================================================================
Age
-----------------------------------------------------------------
<12 months 1-5 years 6-12 years
----------------- --------------- ---------------
Immunologic category cells/uL (%) + cells/uL (%) cells/uL (%)
-------------------------------------------------------------------------------------------------
No evidence of
suppression >=1,500 (>=25) >=1,000 (>=25) >=500 (>=25)
Evidence of moderate
suppression 750-1,499 (15-24) 500-999 (15-24) 200-499 (15-24)
Severe suppression <750 (<15) <500 (<15) <200 (<15)
-------------------------------------------------------------------------------------------------
* Adapted from 1994 revised classification system for human immunodeficiency virus infection
in children aged <13 years (16).
+ Percentage of total lymphocytes.
===================================================================================================
Return to top. Table_2A Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 2A. Prophylaxis for first episode of opportunistic disease in HIV-infected adults
and adolescents
=================================================================================================================================================================
Preventive regimens
-------------------------------------------------------------------------------------
Pathogen Indication First choice Alternatives
---------------------------------------------------------------------------------------------------------------------------------------------------------------
I. Strongly recommended as standard of care
Pneumocystis CD4+ count <200/ uL or Trimethoprim-sulfamethoxazole TMP-SMZ, 1 DS po t.i.w.
carinii* oropharyngeal (TMP-SMZ), 1 DS po q.d. (BIII); dapsone, 50 mg po
candidiasis or (AI); b.i.d. or 100 mg po q.d. (BI);
unexplained fever dapsone, 50 mg po q.d.
>=2 weeks TMP-SMZ, 1 SS po q.d. (AI) plus pyrimethamine, 50 mg
po q.w. plus leucovorin,
25 mg po q.w. (BI);
dapsone, 200 mg po plus
pyrimethamine, 75 mg po
plus leucovorin, 25 mg po
q.w. (BI); aerosolized
pentamidine, 300 mg q.m.
via Respirgard II (TM)
nebulizer (BI)
Mycobacterium
tuberculosis
Isoniazid- TST reaction >=5mm or Isoniazid, 300 mg po plus Rifampin, 600 mg po q.d. x
sensitive + prior positive TST pyridoxine, 50 mg po q.d. x 12 mo (BII)
result without 12 mo (AI) or isoniazid,
treatment or contact 900 mg po plus pyridoxine,
with case of active 50 mg po b.i.w. x 12 mo (BIII)
tuberculosis
Isoniazid- Same; high probability Rifampin, 600 mg po q.d. x Rifabutin, 300 mg po q.d. x
resistant of exposure to 12 mo (BII) 12 mo (CIII)
isoniazid-resistant
tuberculosis
Multidrug- Same; high probability Choice of drugs requires None
(isoniazid and of exposure to consultation with public
rifampin) resistant multidrug-resistant health authorities
tuberculosis
Toxoplasma IgG antibody to TMP-SMZ, 1 DS po q. d. (AII) TMP-SMZ, 1 SS po q.d. (BIII):
gondii & Toxoplasma and CD4+ dapsone, 50 mg po q.d.
count <100/uL plus pyrimethamine, 50 mg
po q.w. plus leucovorin,
25 mg po q.w. (BI)
Mycobacterium CD4+ count <50uL Clarithromycin, 500 mg po Rifabutin, 300 mg po q.d. (BI);
avium complex @ b.i.d. (AI) or azithromycin, azithromycin, 1,200 mg po
1,200 mg po q.w. (AI) q.w. plus rifabutin, 300 mg
po q.d. (CI)
Streptococcus All patients Pneumococcal vaccine, 0.5 mL None
pneumoniae ** im x 1 (CD4+ >=200/uL {AII};
CD4+ <200/uL {CIII})
Varicella zoster Significant exposure to Varicella zoster immune Acyclovir, 800 mg po
virus (VZV) chickenpox or shingles globulin (VZIG), 5 vials 5 times/d for 3 weeks (CIII)
for patients who have (1.25 mL each) im,
no history of either administered <=96 h after
condition or, if exposure, ideally within 48 h
available, negative (AIII)
antibody to VZV
II. Generally recommended
Hepatitis B virus ++ All susceptible Engerix B (R), 20 ug Im x 3 (BII); None
(anti-HBc-negative) or Recombivax HB (R), 10 ug
patients im x 3 (BII)
Influenza virus ++ All patients (annually, Whole or split virus, 0.5 mL Rimantadine, 100 mg po
before influenza im/yr (BIII) b.i.d. (CIII) or amantadine,
season) 100 mg po b.i.d. (CIII)
III. Not recommended for most patients; indicated for use only in unusual circumstances
Candida species CD4+ count <50/ mL Fluconazole, 100-200 mg po
q.d. (CI)
Bacteria Neutropenia Granulocyte-colony-stimulating
factor (G-CSF), 5-10 ug/kg sc
q.d. x 2-4w or
granulocyte-macrophage
colony-stimulating factor
(GM-CSF), 250 ug/m(2) iv over
2 h q.d. x 2-4w (CIII)
Cryptococcus CD4+ count <50/uL Fluconazole, 100-200 mg po Itraconazole, 200 mg po q.d. (CIII)
neoformans && q.d. (CI)
Histoplasma CD4+ count <100/uL, Itraconazole, 200 mg po q.d. None
capsulatum && endemic geographic (CI)
area
Cytomegalovirus CD4+ count <50/uL and Oral ganciclovir, 1 g po t.i.d. None
(CMV) @@ CMV antibody positivity (CI)
---------------------------------------------------------------------------------------------------------------------------------------------------------------
NOTE: Information included in these guidelines may not represent Food and Drug Administration (FDA)
approval or approved labeling for the particular products or indications in question. Specifically, the terms "safe"
and "effective" may not be synonymous with the FDA-defined legal standards for product approval. Anti-HBc =
antibody to hepatitis B core antigen; b.i.w. = twice a week; CMV = cytomegalovirus; DS = double-strength tablet;
q.m. = monthly; q.w. = weekly; SS = single-strength tablet; t.i.w. = three times a week; TMP-SMZ = trimethoprim-
sulfamethoxazole; and TST = tuberculin skin test. The Respirgard II (TM) nebulizer is manufactured by Marquest,
Englewood, CO; Engerix-B (R) by SmithKline Beecham, Rixensart, Belgium; and Recombivax HB (R) by Merck & Co.,
West Point, PA. Letters and Roman numerals in parentheses after regimens indicate the strength of the
recommendation and the quality of evidence supporting it (see text).
* Patients receiving dapsone should be tested for glucose-6 phosphate dehydrogenase deficiency. A dosage of 50
mg q.d. is probably less effective than that of 100 mg q.d. The efficacy of parenteral pentamidine (e.g., 4
mg/kg/month) is uncertain. Inadequate data are available regarding efficacy or safety of atovaquone or
clindamycin-primaquine. Fansidar (sulfadoxine-pyrimethamine) is rarely used because of severe hypersensitivity
reactions. TMP-SMZ reduces the frequency of some bacterial infections. Patients who are being administered
therapy for toxoplasmosis with sulfadiazine-pyrimethamine are protected against Pneumocystis carinii
pneumonia and do not need TMP-SMZ.
+ Directly observed therapy required for isoniazid (INH), 900 mg b.i.w.; INH regimens should include pyridoxine
to prevent peripheral neuropathy. Rifampin should not be administered concurrently with protease inhibitors
(20). Rifabutin, which may be administered at a reduced dose with indinavir or nelfinavir, is an option; consult
an expert. Exposure to multidrug-resistant tuberculosis may require prophylaxis with two drugs; consult public
health authorities. Possible regimens include pyrazinamide plus either ethambutol or a fluoroquinolone (21).
& Protection against Toxoplasma is provided by the preferred anti-Pneumocystis regimens. Pyrimethamine alone
probably provides little, if any, protection.
@ Rifabutin should not be administered concurrently with the protease inhibitors saquinavir or ritonavir; however,
it may be administered at half the dose (150 mg q.d.) with indinavir or nelfinavir.
** Vaccination should be offered to persons who have a CD4+ T-lymphocyte count <200 cells/uL, although the
efficacy may be diminished. Some authorities are concerned that immunizations may stimulate the replication
of HIV. However, one study showed no adverse effect of pneumococcal vaccination on patient survival (22).
Revaccination >=5 years after the first dose is considered optional.
++ These immunizations or chemoprophylactic regimens do not target pathogens traditionally classified as
opportunistic but should be considered for use in HIV-infected patients. Data are inadequate concerning clinical
benefit of these vaccines in this population, although it is logical to assume that those patients who develop
antibody responses will derive some protection. Some authorities are concerned that immunizations may
stimulate HIV replication, although, for influenza vaccination, a large observational study of HIV-infected persons
in clinical care showed no adverse effect of this vaccine, including multiple doses, on patient survival (J. Ward,
CDC, personal communication). Hepatitis B vaccine has been recommended for all children and adolescents and
for all adults with risk factors for hepatitis B infection (23). Rimantadine/amantadine are appropriate during
outbreaks of influenza A. For additional information regarding vaccination against hepatitis B and vaccination
and antiviral therapy against influenza, see references 23 and 24. Because of the theoretical concern that increases
in HIV plasma RNA following vaccination during pregnancy might increase the risk of perinatal transmission of
HIV, providers may wish to defer vaccination until after antiretroviral therapy is initiated.
&& There may be a few unusual occupational or other circumstances under which to consider prophylaxis; consult
a specialist.
@@ Acyclovir is not protective against CMV. Valaciclovir is not recommended because of an unexplained trend toward
increased mortality observed in persons who have AIDS who were being administered this drug for prevention
of CMV disease.
=================================================================================================================================================================
Return to top. Table_2B Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 2B. Prophylaxis for recurrence of opportunistic disease (after chemotherapy
for acute disease) in HIV-infected adults and adolescents
=====================================================================================================================================================
Preventive regimens
--------------------------------------------------------------------------------------
Pathogen Indication First choice Alternatives
---------------------------------------------------------------------------------------------------------------------------------------------------
I. Recommended for life as standard of care
Pneumocystis Prior P. carinii Trimethoprim-sulfamethoxazole TMP-SMZ, 1 DS po t.i.w.. (CIII);
carinii pneumonia (TMP-SMZ), 1 DS po q.d. (AI); dapsone, 50 mg po b.i.d. or
100 mg po q.d. (BI); dapsone,
TMP-SMZ 1 SS po q.d. (AI) 50 mg po q.d. plus
pyrimethamine, 50 mg po q.w.
plus leucovorin, 25 mg po q.w.
(BI); dapsone, 200 mg po plus
pyrimethamine, 75 mg po plus
leucovorin, 25 mg po q.w. (BI);
aerosolized pentamidine, 300 mg
q.m. via Respirgard II (TM)
nebulizer (BI)
Toxoplasma Prior toxoplasmic Sulfadiazine 500-1000 mg po Clindamycin, 300-450 mg po q
gondii * encephalitis q.i.d. plus pyrimethamine 6-8 h plus pyrimethamine,
25-75 mg po q.d. plus 25-75 mg po q.d. plus leucovorin,
leucovorin 10 mg po q.d. (AI) 10-25 mg po q.d.- q.i.d. (BI)
Mycobacterium Documented Clarithromycin, 500 mg po b.i.d. Azithromycin, 500 mg po q.d. (AII)
avium complex + disseminated (AI) plus one or more of the plus one or more of the following:
disease following: ethambutol, ethambutol, 15 mg/kg po q.d.
15 mg/kg po q.d. (AII); (AII); rifabutin, 300 mg po q.d. (AII)
rifabutin, 300 mg po q.d. (AII)
Cytomegalovirus Prior end-organ Ganciclovir, 5-6 mg/kg iv
disease 5-7 days/wk or 1,000 mg po
t.i.d. (AI); or foscarnet,
90-120 mg/kg iv q.d. (AI); or
cidofovir, 5 mg/kg iv q.o.w.
(AI); or (for retinitis)
ganciclovir sustained-release
implant q 6-9 months (AI)
Cryptococcus Documented Fluconazole, 200 mg po q.d. (AI) Amphotericin B, 0.6-1.0 mg/kg iv
neoformans disease q.w.-t.i.w. (AI); itraconazole,
200 mg po q.d. (BI)
Histoplasma Documented Itraconazole, 200 mg po b.i.d. Amphotericin B, 1.0 mg/kg iv q.w.
capsulatum disease (AII) (AII); fluconazole, 400 mg po q.d.
(CII)
Coccidioides Documented Fluconazole, 400 mg po q.d. (AII) Amphotericin B, 1.0 mg/kg iv q.w.
immitis disease (AI); itraconazole, 200 mg po b.i.d.
(AII)
Salmonella Bacteremia Ciprofloxacin, 500 mg po b.i.d. None
species for several months (BII)
(non-typhi) &
II. Recommended only if subsequent episodes are frequent or severe
Herpes simplex Frequent/severe Acyclovir, 200 mg po t.i.d. or
virus recurrences 400 mg po b.i.d. (AI)
Candida (oral, Frequent/severe Fluconazole, 100-200 mg po Ketoconazole, 200 mg po q.d. (CIII);
vaginal, or recurrences q.d. (AI) itraconazole, 200 mg po q.d. (CIII)
esophageal)
---------------------------------------------------------------------------------------------------------------------------------------------------
NOTE: Information included in these guidelines may not represent Food and Drug Administration (FDA)
approval or approved labeling for the particular products or indications in question. Specifically, the terms
"safe" and "effective" may not be synonymous with the FDA-defined legal standards for product approval.
DS = double-strength tablet; q.m. = monthly; q.w. = weekly; SS = single-strength tablet; t.i.w. = three times
a week; and TMP-SMZ = trimethoprim-sulfamethoxazole. The Respirgard II (TM) nebulizer is manufactured by
Marquest, Englewood, CO. Letters and Roman numerals in parentheses after regimens indicate the strength
of the recommendation and the quality of the evidence supporting it (see text).
* Pyrimethamine/sulfadiazine confers protection against PCP as well as toxoplasmosis; clindamycin-
pyrimethamine does not.
+ Many multiple-drug regimens are poorly tolerated. Drug interactions (e.g., those seen with clarithromycin/
rifabutin) can be problematic; rifabutin has been associated with uveitis, especially when administered at
daily doses of >300 mg or concurrently with fluconazole or clarithromycin. Rifabutin should not be admin-
istered concurrently with the protease inhibitors saquinavir or ritonavir, but it can be administered at half
dose (150 mg q.d.) with indinavir or nelfinavir.
& The efficacy of eradication of Salmonella has been demonstrated only for ciprofloxacin.
=====================================================================================================================================================
Return to top. Table_3A Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 3A. Prophylaxis for first episode of opportunistic disease in HIV-infected infants and
children
=========================================================================================================================================================
Preventive regimens
-------------------------------------------------------------------------
Pathogen Indication First choice Alternatives
-------------------------------------------------------------------------------------------------------------------------------------------------------
I. Strongly recommended as standard of care
Pneumocystis HIV-infected or Trimethoprim-sulfamethoxazole Aerosolized pentamidine
carinii * HIV-indeterminate infants (TMP-SMZ), 150/750 mg/m(2)/d (children aged >=5 yr),
aged 1-12 mo; in 2 divided doses po t.i.w. on 300 mg q.m. via Respirgard
consecutive days (AII) II (TM) nebulizer (CIII);
HIV-infected children aged Acceptable alternative dosage dapsone (children aged
1-5 yr with CD4+ count schedules: (AII) >=1 mo), 2 mg/kg (max 100
<500/uL or CD4+ percentage mg) po q.d. (CIII); iv
<15%; pentamidine, 4 mg/kg every
2-4 weeks (CIII)
HIV-infected children aged Single dose po t.i.w. on
6-12 yr with CD4+ count consecutive days;
<200/uL or CD4+ percentage
<15%
2 divided doses po q.d.;
2 divided doses po t.i.w. on
alternate days
Mycobacterium
tuberculosis
Isoniazid- TST reaction >=5mm or prior Isoniazid 10-15 mg/kg (max 300 Rifampin, 10-20 mg/kg (max
sensitive positive TST result without mg) po or im q.d. x 12 mo (AI) 600 mg) po or iv q.d.
treatment or contact with or 20-30 mg/kg (max 900 mg) x 12 mo (BII)
case of active tuberculosis po b.i.w. x 12 mo (BIII)
Isoniazid- Same as above; high Rifampin, 10-20 mg/kg (max Uncertain
resistant probability of exposure to 600 mg) po or iv q.d. x 12 mo
isoniazid-resistant (BII)
tuberculosis
Multidrug Same as above; high Choice of drug requires None
(isoniazid and probability of exposure to consultation with public
rifampin)- multidrug-resistant health authorities
resistant tuberculosis
Mycobacterium For children aged >=6 yrs, Clarithromycin, 7.5 mg/kg (max Children aged >=6 yrs,
avium complex CD4+ count <50/uL; aged 500 mg) po b.i.d. (AII), or rifabutin, 300 mg po q.d.
2-6 yrs, CD4+ count <75/uL; azithromycin, 20 mg/kg (max (BI); children aged <6 yrs,
aged 1-2 yrs, CD4+ count 1,200 mg) po q.w. (AII) 5 mg/kg po q.d. when
<500/uL; aged <1 yr, CD4+ suspension becomes
count <750/uL available (BI); azithromycin,
5 mg/kg (max 250 mg) po
q.d. (AII)
Varicella zoster Significant exposure to Varicella zoster immune None
virus + varicella with no history of globulin (VZIG), 1 vial
chickenpox or shingles (1.25 mL)/10 kg (max 5 vials)
im, administered <=96 hrs after
exposure, ideally within
48 hrs (AII)
Vaccine- HIV exposure/ infection Routine immunizations (see None
preventable Figure)
pathogens &
II. Generally recommended
Toxoplasma IgG antibody to Toxoplasma TMP-SMZ, 150/750 mg/m(2)/d in Dapsone (children aged
gondii @ and severe 2 divided doses po q.d. (BIII) >=1 mo), 2 mg/kg or
immunosuppression 15 mg/m(2) (max 25 mg) po
q.d. plus pyrimethamine,
1 mg/kg po q.d. plus
leucovorin, 5 mg po every
3 days (BIII)
III. Not recommended for most patients; indicated for use only in unusual circumstances
Invasive bacterial Hypogammaglobulinemia IVIG (400 mg/kg/q.m.) (AI) None
infections **
Candida species Severe immunosuppression Nystatin (100,000 U/mL), None
4-6 mL po q 6 h (CIII) or
topical clotrimazole, 10 mg po
5x/ d (CIII)
Cryptococcus Severe immunosuppression Fluconazole, 3-6 mg/kg po q.d. Itraconazole, 2-5 mg/kg po q
neoformans (CII) 12-24 h (CIII)
Histoplasma Severe immunosuppression, Itraconazole, 2-5 mg/kg po None
capsulatum endemic geographic area q 12-24 h (CII)
Cytomegalovirus CMV antibody positivity and Children aged 6-12 yrs: oral None
(CMV) ++ severe immunosuppression ganciclovir under investigation
-------------------------------------------------------------------------------------------------------------------------------------------------------
NOTE: Information included in these guidelines may not represent Food and Drug Administration (FDA)
approval or approved labeling for the particular products or indications in question. Specifically, the terms
"safe" and "effective" may not be synonymous with the FDA-defined legal standards for product approval.
b.i.w. = twice a week; CMV = cytomegalovirus; IVIG = intravenous immune globulin; q.m. = monthly; t.i.w. =
three times a week; TMP-SMZ = trimethoprim-sulfamethoxazole; and VZIG = varicella zoster immune
globulin. The Respirgard II (TM) nebulizer is manufactured by Marquest, Englewood, CO. Letters and Roman
numerals in parentheses after regimens indicate the strength of the recommendation and the quality of the
evidence supporting it (see text).
* The efficacy of parenteral pentamidine (e.g., 4 mg/kg/month) is controversial. TMP-SMZ, dapsone-
pyrimethamine, and possibly dapsone alone appear to protect against toxoplasmosis, although data have
not been prospectively collected. Daily TMP-SMZ reduces the frequency of some bacterial infections. Patients
receiving therapy for toxoplasmosis with sulfadiazine-pyrimethamine are protected against Pneumocystis
carinii pneumonia (PCP) and do not need TMP-SMZ.
+ Children routinely being administered intravenous immune globulin (IVIG) should receive VZIG if the last
dose of IVIG was administered >21 days before exposure.
& HIV-infected and HIV-exposed children should be immunized according to the following childhood
immunization schedule (Figure), which has been adapted from the January-December 1997 schedule
recommended for immunocompetent children by the Advisory Committee on Immunization Practices, the
American Academy of Pediatrics, and the American Academy of Family Physicians. This schedule differs
from that for immunocompetent children in that IPV replaces OPV, vaccination against S. pneumoniae (AII)
and influenza (BIII) should be offered, and vaccination against varicella is contraindicated (EIII). MMR should
not be administered to severely immunocompromised children (DIII). Once an HIV-exposed child is
determined not to be HIV infected, the schedule for immunocompetent children applies.
@ Protection against Toxoplasma is provided by the preferred antipneumocystis regimens. Pyrimethamine
alone probably provides little, if any, protection. For definition of severe immunosuppression, see Table 1.
** Respiratory syncytial virus (RSV) IVIG may be substituted for IVIG during the RSV season.
++ Data on oral ganciclovir are still being evaluated; durability of effect is unclear. Acyclovir is not protective
against CMV.
=========================================================================================================================================================
Return to top. Figure_1A 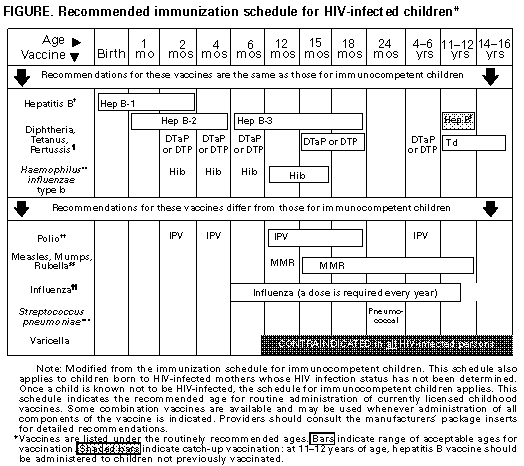 Return to top. Figure_1B  Return to top. Table_3B Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 3B. Prophylaxis for recurrence of opportunistic disease (after chemotherapy for
acute disease) in HIV-infected infants and children
=======================================================================================================================================
Preventive regimens
--------------------------------------------------------------------
Pathogen Indication First choice Alternative
-------------------------------------------------------------------------------------------------------------------------------------
I. Recommended for life as standard of care
Pneumocystis carinii Prior P. carinii TMP-SMZ, 150/750 Aerosolized pentamidine
pneumonia mg/m(2)/d in 2 divided (children aged >=5 yrs),
doses po t.i.w. on 300 mg q.m. via
consecutive days (AII) Respirgard II (TM)
nebulizer (CIII); dapsone
Acceptable alternative (children aged >=1 mo), 2
schedules for same mg/kg (max 100 mg) po
dosage: (AII) q.d. (CIII); iv pentam-
Single dose po t.i.w. on idine, 4 mg/kg every 2-4
consecutive days; weeks (CIII)
2 divided doses po q.d.;
2 divided doses po t.i.w.
on alternate days
Toxoplasma gondii * Prior toxoplasmic Sulfadiazine, Clindamycin,
encephalitis 85-120 mg/kg/d in 20-30 mg/kg/d in
2-4 divided doses po 4 divided doses po q.d.
q.d. plus plus pyrimethamine,
pyrimethamine, 1 mg/kg 1 mg/kg po q.d. plus
or 15 mg/m(2) (max leucovorin, 5 mg po
25 mg) po q.d. plus every 3 days (BI)
leucovorin, 5 mg po
every 3 days (AI)
Mycobacterium avium Prior disease Clarithromycin, 7.5 mg/kg
complex (max 500 mg) po b.i.d.
(AII) plus at least one of
the following:
ethambutol, 15 mg/kg
(max 900 mg) po q.d.
(AII); rifabutin, 5 mg/kg
(max 300 mg) po q.d.
(AII)
Cryptococcus Documented disease Fluconazole, 3-6 mg/kg po Itraconazole, 2-5 mg/kg
neoformans q.d. (AII) po q 24-12h (BII);
amphotericin B,
0.5-1.5 mg/kg iv
1-3x/ week (AI)
Histoplasma capsulatum Documented disease Itraconazole, 2-5 mg/kg Fluconazole, 3-6 mg/kg po
po q 12-48h (AIII) q.d. (CIII); amphotericin
B, 1.0 mg/kg iv q.w. AIII)
Coccidioides immitis Documented disease Fluconazole, 6 mg/kg po Amphotericin B,
q.d. (AIII) 1.0 mg/kg iv q.w. (AII)
Cytomegalovirus Prior end-organ disease Ganciclovir, 5 mg/kg iv
q.d.; or foscarnet,
90-120 mg/kg iv q.d.
(AI); or (for retinitis)
ganciclovir
sustained-release
implant (AI)
Salmonella species Bacteremia TMP- SMZ, 150/750 mg/m(2) Antibiotic chemo-
(non-typhi) + in 2 divided doses po prophylaxis with
q.d. for several months another active agent
(CIII) (CIII)
II. Recommended only if subsequent episodes are frequent or severe
Invasive bacterial >2 infections in 1-year TMP-SMZ, 150/750 mg/ m(2) Antibiotic chemo-
infections & period in 2 divided doses po prophylaxis with
q.d. (BI); or IVIG, another active agent
400 mg/kg q.m. (BI) (BIII)
Herpes simplex virus Frequent/severe Acyclovir, 80 mg/kg/d in
recurrences 3-4 divided doses po
q.d. (AII)
Candida species Frequent/severe Fluconazole, 3-6 mg/kg po
recurrences q.d. (AII); or
ketoconazole, 5-10
mg/kg po q 24-12h (CIII)
-------------------------------------------------------------------------------------------------------------------------------------
NOTE: Information included in these guidelines may not represent Food and Drug Administration (FDA)
approval or approved labeling for the particular products or indications in question. Specifically, the terms
"safe" and "effective" may not be synonymous with the FDA-defined legal standards for product approval.
IVIG = intravenous immune globulin; q.m.= monthly; q.w. = weekly; t.i.w. = three times a week; and
TMP-SMZ = trimethoprim-sulfamethoxazole. The Respirgard II (TM) nebulizer is manufactured by Marquest,
Englewood, CO. Letters and Roman numerals in parentheses after regimens indicate the strength of the
recommendations and the quality of the evidence supporting it (see text).
* Only pyrimethamine plus sulfadiazine confers protection against PCP as well as toxoplasmosis. Although
the clindamycin plus pyrimethamine regimen is the preferred alternative in adults, it has not been tested
in children. However, these drugs are safe and are used for other infections.
+ Drug should be determined by susceptibilities of the organism isolated. Alternatives to TMP-SMZ include
ampicillin, chloramphenicol, or ciprofloxacin. However, ciprofloxacin is not approved for use in persons
aged <18 years; therefore, it should be used in children with caution and only if no alternatives exist.
& Antimicrobial prophylaxis should be chosen based on the microorganism and antibiotic sensitivities.
TMP-SMZ, if used, should be administered daily. Providers should be cautious about using antibiotics solely
for this purpose because of the potential for development of drug-resistant microorganisms. IVIG may not
provide additional benefit to children receiving daily TMP-SMZ. Choice of antibiotic prophylaxis vs. IVIG
should also involve consideration of adherence, ease of intravenous access, and cost. If IVIG is used, RSV-IVIG
may be substituted for IVIG during the RSV season.
=======================================================================================================================================
Return to top. Table_4 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
Table 4. Advising patients concerning prevention of exposure to
opportunistic pathogens
===========================================================================
Sexual Exposures
---------------------------------------------------------------------------
(1) Patients should use a latex condom during every act of sexual
intercourse to reduce the risk of acquisition of cytomegalovirus,
herpes simplex virus, and human papillomavirus, as well as other
sexually transmitted pathogens (AII). Condom use also will,
theoretically, reduce the risk of acquisition of human herpesvirus
8, as well as superinfection with an HIV strain that has become
resistant to antiretroviral drugs (BIII) and will prevent
transmission of HIV and other sexually transmitted pathogens to
others (AII). Data regarding the use and efficacy of "female
condoms" are incomplete, but these devices should be considered as
a risk-reduction strategy (BIII).
(2) Patients should avoid sexual practices that may result in oral
exposure to feces (e.g., oral-anal contact) to reduce the risk of
intestinal infections (e.g., cryptosporidiosis, shigellosis,
campylobacteriosis, amebiasis, giardiasis, and hepatitis A and B)
(BIII).
Environmental and Occupational Exposures
(1) Certain activities or types of employment may increase the
risk of exposure to tuberculosis (BIII). These include volunteer
work or employment in health-care facilities, correctional
institutions, and shelters for the homeless, as well as other
settings identified as high risk by local health authorities.
Decisions about whether to continue with such activities should be
made in conjunction with the health-care provider and should be
based on such factors as the patient's specific duties in the
workplace, the prevalence of tuberculosis in the community, and
the degree to which precautions designed to prevent the
transmission of tuberculosis are taken in the workplace (BIII).
These decisions will affect the frequency with which the patient
should be screened for tuberculosis.
(2) Child care providers and parents of children in child care are
at increased risk of acquiring CMV infection, cryptosporidiosis,
and other infections (e.g., hepatitis A and giardiasis) from
children. The risk of acquiring infection can be diminished by
good hygienic practices, such as hand washing after fecal contact
(e.g., during diaper changing and after contact with urine or
saliva) (AII). All children in child care facilities also are at
increased risk of acquiring these same infections; parents and
other caretakers of HIV-infected children should be advised of
this risk (BIII).
(3) Occupations involving contact with animals (e.g., veterinary
work and employment in pet stores, farms, or slaughterhouses) may
pose a risk of cryptosporidiosis, toxoplasmosis, salmonellosis,
campylobacteriosis, or Bartonella infection. However, the
available data are insufficient to justify a recommendation
against work in such settings.
(4) Contact with young farm animals, especially animals with
diarrhea, should be avoided to reduce the risk of
cryptosporidiosis (BII).
(5) Hand washing after gardening or other contact with soil may
reduce the risk of cryptosporidiosis and toxoplasmosis (BIII).
(6) In areas endemic for histoplasmosis, patients should avoid
activities known to be associated with increased risk, including
cleaning chicken coops, disturbing soil beneath bird-roosting
sites, and exploring caves (CIII).
(7) In areas endemic for coccidioidomycosis, when possible,
patients should avoid activities associated with increased risk,
including those involving extensive exposure to disturbed native
soil (e.g., at excavation sites or during dust storms) (CIII).
Pet-Related Exposures
Health-care providers should advise HIV-infected persons of the
potential risk posed by pet ownership. However, they should be
sensitive to the possible psychological benefits of pet ownership
and should not routinely advise HIV-infected persons to part with
their pets (DIII). Specifically, providers should advise
HIV-infected patients of the following.
General
(1) Veterinary care should be sought when a pet develops diarrheal
illness. If possible, HIV-infected persons should avoid contact
with animals that have diarrhea (BIII). A fecal sample should be
obtained from animals with diarrhea and examined for
Cryptosporidium, Salmonella, and Campylobacter.
(2) When obtaining a new pet, HIV-infected patients should avoid
animals aged less than 6 months, especially those with diarrhea
(BIII). Because the hygienic and sanitary conditions in
pet-breeding facilities, pet stores, and animal shelters are
highly variable, the patient should be cautious when obtaining a
pet from these sources. Stray animals should be avoided. Animals
aged less than 6 months, especially those with diarrhea, should be
examined by a veterinarian for Cryptosporidium, Salmonella, and
Campylobacter (BIII).
(3) Patients should wash their hands after handling pets
(especially before eating) and avoid contact with pets' feces to
reduce the risk of cryptosporidiosis, salmonellosis, and
campylobacteriosis (BIII). Hand washing for HIV-infected children
should be supervised.
Cats
(4) Patients should consider the potential risks of cat ownership
because of the risks of toxoplasmosis and Bartonella infection, as
well as enteric infections (CIII). Those who elect to obtain a cat
should adopt or purchase an animal that is aged greater than 1
year and in good health to reduce the risk of cryptosporidiosis,
Bartonella infection, salmonellosis, and campylobacteriosis (BII).
(5) Litter boxes should be cleaned daily, preferably by an
HIV-negative, nonpregnant person; if the HIV-infected patient
performs this task, he or she should wash hands thoroughly
afterward to reduce the risk of toxoplasmosis (BIII).
(6) To reduce the risk of toxoplasmosis, cats should be kept
indoors, should not be allowed to hunt, and should not be fed raw
or undercooked meat (BIII).
(7) Although declawing is not generally advised, patients should
avoid activities that may result in cat scratches or bites to
reduce the risk of Bartonella infection (BII). Patients should
also wash sites of cat scratches or bites promptly (CIII) and
should not allow cats to lick open cuts or wounds (BIII).
(8) Care of cats should include flea control to reduce the risk of
Bartonella infection (CIII).
(9) Testing cats for toxoplasmosis (EII) or Bartonella infection
(DII) is not recommended.
Birds
(10) Screening healthy birds for Cryptococcus neoformans,
Mycobacterium avium, or Histoplasma capsulatum is not recommended
(DIII).
Other
(11) Contact with reptiles (e.g., snakes, lizards, iguanas, and
turtles) should be avoided to reduce the risk of salmonellosis
(BIII).
(12) Gloves should be used during the cleaning of aquariums to
reduce the risk of infection with Mycobacterium marinum (BIII).
(13) Contact with exotic pets (e.g., nonhuman primates) should be
avoided (CIII).
Food- and Water-Related Exposures
(1) Raw or undercooked eggs (including foods that may contain raw
eggs {e.g., some preparations of hollandaise sauce, Caesar and
certain other salad dressings, and mayonnaise}); raw or
undercooked poultry, meat, seafood; and unpasteurized dairy
products may contain enteric pathogens. Poultry and meat should be
cooked until no longer pink in the middle (internal temperature,
greater than 165 F {73.8 C}). Produce should be washed thoroughly
before being eaten (BIII).
(2) Cross-contamination of foods should be avoided. Uncooked meats
should not be allowed to come in contact with other foods; hands,
cutting boards, counters, and knives and other utensils should be
washed thoroughly after contact with uncooked foods (BIII).
(3) Although the incidence of listeriosis is low, it is a serious
disease that occurs unusually frequently among HIV-infected
persons who are severely immunosuppressed. Some soft cheeses and
some ready-to-eat foods (e.g., hot dogs and cold cuts from
delicatessen counters) have been known to cause listeriosis. An
HIV-infected person who is severely immunosuppressed and who
wishes to reduce the risk of foodborne disease can prevent
listeriosis by reheating these foods until they are steaming
before eating them (CIII).
(4) Patients should not drink water directly from lakes or rivers
because of the risk of cryptosporidiosis and giardiasis (AIII).
Waterborne infection may also result from swallowing water during
recreational activities. Patients should avoid swimming in water
that is likely to be contaminated with human or animal waste and
should avoid swallowing water during swimming (BII).
(5) During outbreaks or in other situations in which a community
"boil water advisory" is issued, boiling water for 1 minute will
eliminate the risk of acquiring cryptosporidiosis (AI). Using
submicron, personal-use water filters (home/ office types) and/or
drinking bottled water* may reduce the risk (CIII). Current data
are inadequate to support a recommendation that all HIV-infected
persons boil or otherwise avoid drinking tap water in nonoutbreak
settings. However, persons who wish to take independent action to
reduce their risk of waterborne cryptosporidiosis may choose to
take precautions similar to those recommended during outbreaks.
Such decisions are best made in conjunction with a health-care
provider. Persons who opt for a personal-use filter or bottled
water should be aware of the complexities involved in selecting
the appropriate products, the lack of enforceable standards for
destruction or removal of oocysts, the cost of the products, and
the difficulty of using these products consistently. Patients
taking precautions to avoid acquiring cryptosporidiosis from
drinking water should be advised that ice made from contaminated
tap water also can be a source of infection (BII). Such persons
should be aware that fountain beverages served in restaurants,
bars, theaters, and other public places may also pose a risk,
because these beverages, as well as the ice they may contain, are
made from tap water. Nationally distributed brands of bottled or
canned carbonated soft drinks are safe to drink. Commercially
packaged noncarbonated soft drinks and fruit juices that do not
require refrigeration until after they are opened (e.g., those
that can be stored unrefrigerated on grocery shelves) also are
safe. Nationally distributed brands of frozen fruit juice
concentrate are safe if they are reconstituted by the user with
water from a safe source. Fruit juices that must be kept
refrigerated from the time they are processed to the time of
consumption may be either fresh (unpasteurized) or heat treated
(pasteurized); only juices labeled as pasteurized should be
considered free of risk from Cryptosporidium. Other pasteurized
beverages and beers are also considered safe to drink (BII). No
data are available concerning survival of Cryptosporidium oocysts
in wine.
Travel-Related Exposures
(1) Travel, particularly to developing countries, may carry
significant risks for the exposure of HIV-infected persons to
opportunistic pathogens, especially for patients who are severely
immunosuppressed. Consultation with health-care providers and/or
with experts in travel medicine will help patients plan
itineraries (BIII).
(2) During travel to developing countries, HIV-infected persons
are at even higher risk for foodborne and waterborne infections
than they are in the United States. Foods and beverages--in
particular, raw fruits and vegetables, raw or undercooked seafood
or meat, tap water, ice made with tap water, unpasteurized milk
and dairy products, and items purchased from street vendors--may
be contaminated (AII). Items that are generally safe include
steaming-hot foods, fruits that are peeled by the traveler,
bottled (especially carbonated) beverages, hot coffee or tea,
beer, wine, and water brought to a rolling boil for 1 minute
(AII). Treating water with iodine or chlorine may not be as
effective as boiling but can be used, perhaps in conjunction with
filtration, when boiling is not practical (BIII).
(3) Waterborne infections may result from swallowing water during
recreational activities. To reduce the risk of cryptosporidiosis
and giardiasis, patients should avoid swallowing water during
swimming and should not swim in water that may be contaminated
(e.g., with sewage or animal waste) (BII).
(4) Antimicrobial prophylaxis for traveler's diarrhea is not
recommended routinely for HIV-infected persons traveling to
developing countries (DIII). Such preventive therapy can have
adverse effects and can promote the emergence of drug-resistant
organisms. Nonetheless, several studies (none involving an
HIV-infected population) have shown that prophylaxis can reduce
the risk of diarrhea among travelers. Under selected circumstances
(e.g., those in which the risk of infection is very high and the
period of travel brief), the provider and patient may weigh the
potential risks and benefits and decide that antibiotic
prophylaxis is warranted (CIII). For those persons to whom
prophylaxis is offered, fluoroquinolones (e.g., ciprofloxacin {500
mg q.d.}) can be considered (BIII). Trimethoprim-sulfamethoxazole
(TMP-SMZ) (one double-strength tablet daily) also has been shown
to be effective, but resistance to this drug is now common in
tropical areas. Persons already taking TMP-SMZ for prophylaxis
against Pneumocystis carinii pneumonia (PCP) may gain some
protection against traveler's diarrhea. For HIV-infected persons
who are not already taking TMP-SMZ, health-care providers should
be cautious in prescribing this agent for prophylaxis of diarrhea
because of the high rates of adverse reactions and the possible
need for the agent for other purposes (e.g., PCP prophylaxis) in
the future.
(5) All HIV-infected travelers to developing countries should
carry a sufficient supply of an antimicrobial agent to be taken
empirically should diarrhea develop (BIII). One appropriate
regimen is 500 mg of ciprofloxacin b.i.d. for 3-7 days.
Alternative antibiotics (e.g., TMP-SMZ) should be considered as
empirical therapy for use by children and pregnant women (CIII).
Travelers should consult a physician if their diarrhea is severe
and does not respond to empirical therapy, if their stools contain
blood, if fever is accompanied by shaking chills, or if
dehydration develops. Antiperistaltic agents (e.g., diphenoxylate
and loperamide) are used for the treatment of diarrhea; however,
they should not be used by patients with high fever or with blood
in the stool, and their use should be discontinued if symptoms
persist beyond 48 hours (AII). These drugs are not recommended for
children (DIII).
(6) Travelers should be advised about other preventive measures
appropriate for anticipated exposures (e.g., chemoprophylaxis for
malaria, protection against arthropod vectors, treatment with
immune globulin, and vaccination) (AII). They should avoid direct
contact of the skin with soil or sand (e.g., by wearing shoes and
protective clothing and using towels on beaches) in areas where
fecal contamination of soil is likely (BIII).
(7) In general, live-virus vaccines should be avoided (EII). An
exception is measles vaccine, which is recommended for nonimmune
persons. However, measles vaccine is not recommended for those who
are severely immunosuppressed (DIII); immune globulin should be
considered for measles-susceptible, severely immunosuppressed
persons who are anticipating travel to measles-endemic countries
(BIII). Inactivated (killed) poliovirus vaccine should be used
instead of oral (live) poliovirus vaccine, which is
contraindicated for HIV-infected persons. Persons at risk for
exposure to typhoid fever should be administered an inactivated
parenteral typhoid vaccine instead of the live attenuated oral
preparation. Yellow fever vaccine is a live-virus vaccine with
uncertain safety and efficacy in HIV-infected persons. Travelers
with asymptomatic HIV infection who cannot avoid potential
exposure to yellow fever should be offered the choice of
vaccination. If travel to a zone with yellow fever is necessary
and vaccination is not administered, patients should be advised of
the risk, instructed in methods for avoiding the bites of vector
mosquitoes, and provided with a vaccination waiver letter.
(8) In general, killed vaccines (e.g., diphtheria-tetanus, rabies,
hepatitis A, Japanese encephalitis vaccines) should be used for
HIV-infected persons just as they would be used for
non-HIV-infected persons anticipating travel (BIII). Preparation
for travel should include a review and updating of routine
vaccinations, including diphtheria-tetanus for adults and all
routine immunizations for children. The currently available
cholera vaccine is not recommended for persons following a usual
tourist itinerary, even if travel includes countries reporting
cases of cholera (DII).
(9) Travelers should be informed about other area-specific risks
and instructed in ways to reduce those risks (BIII).
Geographically focal infections that pose a high risk to
HIV-infected persons include visceral leishmaniasis (a protozoan
infection transmitted by the sandfly) and several fungal
infections (e.g., Penicillium marneffei infection,
coccidioidomycosis, and histoplasmosis). Many tropical and
developing areas have high rates of tuberculosis.
===========================================================================
Return to top. Table_5 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 5. Wholesale acquisition cost of agents recommended for prevention of
opportunistic infections in patients who have HIV infection
======================================================================================================================
Opportunistic
Pathogen Drug Dose Annual Cost
--------------------------------------------------------------------------------------------------------------------
Pneumocystis carinii Trimethoprim-sulfamethoxazole 160/800 mg q.d. $60
Dapsone 100 mg q.d. $72
Aerosolized pentamidine 300 mg q.m. $1,185
Mycobacterium Rifabutin 300 mg q.d. $ 2,916
avium complex Clarithromycin 500 mg b.i.d. $ 2,347
Azithromycin 1,200 mg q.w. $1,490
Cytomegalovirus Ganciclovir (iv) 5 mg/kg q.d. $8,891
Foscarnet (iv) 90-120 mg/kg q.d. $27,800-37,200
Ganciclovir (po) 1,000 mg t.i.d. $17,082
Cidofovir (iv) 375 mg q.o.w. $17,670
Ganciclovir implant $5, 000
Mycobacterium Isoniazid 300 mg q.d. $31
tuberculosis Rifampin 600 mg q.d. $1,542
Pyrazinamide 1,500 mg q.d. $1,187
Ethambutol 900 mg q.d. $1,370
Fungi Fluconazole 200 mg q.d. $5,018
Itraconazole 200 mg q.d. $4,239
Ketoconazole 200 mg q.d. $1,069
Herpes simplex virus Acyclovir 400 mg t.i.d. $2,377
Toxoplasma gondii Pyrimethamine 500 mg q.w. $43
Leucovorin 25 mg q.w. $727
Cost per
Pathogen Vaccine Dose dose listed
--------------------------------------------------------------------------------------------------------------------
Streptococcus
pneumoniae 23-valent pneumococcal 0.5 mL im $15
Influenza virus Influenza vaccine 0.5 mL im $5
Hepatitis B virus Recombinant hepatitis B 10-20 ug im x 3 $173
--------------------------------------------------------------------------------------------------------------------
Source: Drug Topics Red Book, Medical Economics Inc., Montvale, NJ, 1997.
======================================================================================================================
Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|